Production of capsular polysaccharide does not influence Staphylococcus aureus vancomycin susceptibility
- PMID: 23522028
- PMCID: PMC3617075
- DOI: 10.1186/1471-2180-13-65
Production of capsular polysaccharide does not influence Staphylococcus aureus vancomycin susceptibility
Abstract
Background: Diverse mechanisms (increased cell wall thickness, low cross linking, decreased autolysis, etc.) have been reported for Staphylococcus aureus strains with intermediate vancomycin susceptibility (VISA). This study was conducted to identify common mechanisms responsible for decreased vancomycin susceptibility in a VISA strain pair.
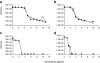
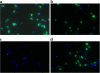
Results: Transcriptional profiling of the clinical heterogeneous VISA isolate SA137/93A and its spontaneous homogeneous mutant strain SA137/93G pointed to an increased capsule production in the strain pair compared to a susceptible control. Furthermore, transcript quantification of the gene cap5E, which is essential for capsule biosynthesis, revealed elevated levels in the VISA strains SA137/93A, SA137/93G and Mu50 in comparison with susceptible strains Reynolds, Newman and SA1450/94. The increased expression was observed in bacteria from exponential as well as stationary growth phase. However, suppression of type 5 capsule formation by expression of antisense RNA did not increase vancomycin susceptibility in the VISA strain SA137/93G. Likewise, construction of inducible mutants of S. aureus Newman or repair of capsule biosynthesis of S. aureus HG001 and S. aureus 1450/94 did not influence resistance to vancomycin. Furthermore, purified type 5 polysaccharide did not protect indicator strains from the action of vancomycin.
Conclusions: The VISA strain tested in this study displayed an increased production of type 5 capsular polysaccharide. However, the production of capsule material did not protect strain SA137/93G and three vancomycin sensitive strains in the presence of vancomycin and thus is not part of the resistance mechanism; however it may represent a by-product of VISA life style that is often characterized by a high sigma factor B activity.
Figures





References
-
- Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42:199–209. doi: 10.1093/jac/42.2.199. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

