The θ-γ neural code
- PMID: 23522038
- PMCID: PMC3648857
- DOI: 10.1016/j.neuron.2013.03.007
The θ-γ neural code
Abstract
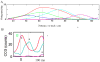
Theta and gamma frequency oscillations occur in the same brain regions and interact with each other, a process called cross-frequency coupling. Here, we review evidence for the following hypothesis: that the dual oscillations form a code for representing multiple items in an ordered way. This form of coding has been most clearly demonstrated in the hippocampus, where different spatial information is represented in different gamma subcycles of a theta cycle. Other experiments have tested the functional importance of oscillations and their coupling. These involve correlation of oscillatory properties with memory states, correlation with memory performance, and effects of disrupting oscillations on memory. Recent work suggests that this coding scheme coordinates communication between brain regions and is involved in sensory as well as memory processes.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
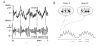
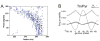
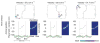



References
-
- Adrian ED, Matthews BHC. The Berger rhythm: potential changes from the occipital lobes in man. Brain. 1934;57:355–385. - PubMed
-
- Alonso A, Khateb A, Fort P, Jones BE, Muhlethaler M. Differential oscillatory properties of cholinergic and noncholinergic nucleus basalis neurons in guinea pig brain slice. Eur J Neurosci. 1996;8:169–182. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

