Regulation of postsynaptic retrograde signaling by presynaptic exosome release
- PMID: 23522040
- PMCID: PMC3626103
- DOI: 10.1016/j.neuron.2013.01.013
Regulation of postsynaptic retrograde signaling by presynaptic exosome release
Abstract
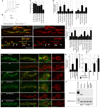
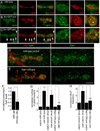
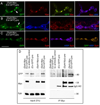
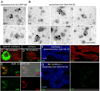
Retrograde signals from postsynaptic targets are critical during development and plasticity of synaptic connections. These signals serve to adjust the activity of presynaptic cells according to postsynaptic cell outputs and to maintain synaptic function within a dynamic range. Despite their importance, the mechanisms that trigger the release of retrograde signals and the role of presynaptic cells in this signaling event are unknown. Here we show that a retrograde signal mediated by Synaptotagmin 4 (Syt4) is transmitted to the postsynaptic cell through anterograde delivery of Syt4 via exosomes. Thus, by transferring an essential component of retrograde signaling through exosomes, presynaptic cells enable retrograde signaling.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Bai J, Sepp KJ, Perrimon N. Culture of Drosophila primary cells dissociated from gastrula embryos and their use in RNAi screening. Nat Protoc. 2009;4:1502–1512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

