Who benefits from adjuvant radiation therapy for gastric cancer? A meta-analysis
- PMID: 23523184
- PMCID: PMC3817859
- DOI: 10.1016/j.ijrobp.2013.02.008
Who benefits from adjuvant radiation therapy for gastric cancer? A meta-analysis
Abstract
Purpose: Large randomized trials have demonstrated significant survival benefits with the use of adjuvant chemotherapy or chemoradiation therapy for gastric cancer. The importance of adjuvant radiation therapy (RT) remains unclear. We performed an up-to-date meta-analysis of randomized trials testing the use of RT for resectable gastric cancer.
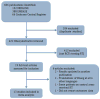
Methods and materials: We searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials for randomized trials testing adjuvant (including neoadjuvant) RT for resectable gastric cancer. Hazard ratios describing the impact of adjuvant RT on overall survival (OS) and disease-free survival (DFS) were extracted directly from the original studies or calculated from survival curves. Pooled estimates were obtained using the inverse variance method. Subgroup analyses were performed to determine whether the efficacy of RT varies with chemotherapy use, RT timing, geographic region, type of nodal dissection performed, or lymph node status.
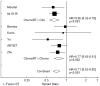
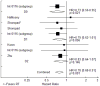
Results: Thirteen studies met all inclusion criteria and were used for this analysis. Adjuvant RT was associated with a significant improvement in both OS (HR = 0.78, 95% CI: 0.70-0.86, P<.001) and DFS (HR = 0.71, 95% CI: 0.63-0.80, P<.001). In the 5 studies that tested adjuvant chemoradiation therapy against adjuvant chemotherapy, similar effects were seen for OS (HR = 0.83, 95% CI: 0.67-1.03, P=.087) and DFS (HR = 0.77, 95% CI: 0.91-0.65, P=.002). Available data did not reveal any subgroup of patients that does not benefit from adjuvant RT.
Conclusion: In randomized trials for resectable gastric cancer, adjuvant RT provides an approximately 20% improvement in both DFS and OS. Available data do not reveal a subgroup of patients that does not benefit from adjuvant RT. Further study is required to optimize the implementation of adjuvant RT for gastric cancer with regard to patient selection and integration with systemic therapy.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors have no conflicts of interest to disclose.
Figures


References
-
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1893–1907. - PubMed
-
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European organisation for research and treatment of cancer randomized trial 40954. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:5210–5218. - PMC - PubMed
-
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine. 2006;355:11–20. - PubMed
-
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of swog-directed intergroup study 0116: A phase iii trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2327–2333. - PMC - PubMed
-
- Bamias A, Karina M, Papakostas P, et al. A randomized phase iii study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer chemotherapy and pharmacology. 2010;65:1009–1021. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

