Structure of the catalytic region of DNA ligase IV in complex with an Artemis fragment sheds light on double-strand break repair
- PMID: 23523427
- PMCID: PMC3664939
- DOI: 10.1016/j.str.2013.02.014
Structure of the catalytic region of DNA ligase IV in complex with an Artemis fragment sheds light on double-strand break repair
Abstract
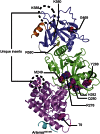
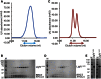
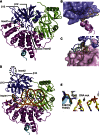
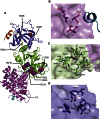
Nonhomologous end joining (NHEJ) is central to the repair of double-stranded DNA breaks throughout the cell cycle and plays roles in the development of the immune system. Although three-dimensional structures of most components of NHEJ have been defined, those of the catalytic region of DNA ligase IV (LigIV), a specialized DNA ligase known to work in NHEJ, and of Artemis have remained unresolved. Here, we report the crystal structure at 2.4 Å resolution of the catalytic region of LigIV (residues 1-609) in complex with an Artemis peptide. We describe interactions of the DNA-binding domain of LigIV with the continuous epitope of Artemis, which, together, form a three-helix bundle. A kink in the first helix of LigIV introduced by a conserved VPF motif gives rise to a hydrophobic pocket, which accommodates a conserved tryptophan from Artemis. We provide structural insights into features of LigIV among human DNA ligases.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

