Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy
- PMID: 23523428
- PMCID: PMC3645870
- DOI: 10.1016/j.biomaterials.2013.02.063
Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy
Abstract
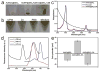
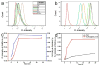
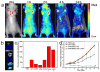
Multifunctional theranostics have recently been intensively explored to optimize the efficacy and safety of therapeutic regimens. In this work, a photo-theranostic agent based on chlorin e6 (Ce6) photosensitizer-conjugated silica-coated gold nanoclusters (AuNCs@SiO2-Ce6) is strategically designed and prepared for fluorescence imaging-guided photodynamic therapy (PDT). The AuNCs@SiO2-Ce6 shows the following features: i) high Ce6 photosensitizer loading; ii) no non-specific release of Ce6 during its circulation; iii) significantly enhanced cellular uptake efficiency of Ce6, offering a remarkably improved photodynamic therapeutic efficacy compared to free Ce6; iv) subcellular characterization of the nanoformula via both the fluorescence of Ce6 and plasmon luminescence of AuNCs; v) fluorescence imaging-guided photodynamic therapy (PDT). This photo-theranostics owns good stability, high water dispersibility and solubility, non-cytotoxicity, and good biocompatibility, thus facilitating its biomedical applications, particularly for multi-modal optical, CT and photoacoustic (PA) imaging-guided PDT or sonodynamic therapy.
Published by Elsevier Ltd.
Figures





References
-
- Lovell J, Jin C, Huynh E, Jin H, Kim C, Rubinstein J, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;20:324–32. - PubMed
-
- Lovell J, Liu T, Chen J, Zheng G. Activatable photosensitizers for imaging and therapy. Chem Rev. 2010;110:2839–57. - PubMed
-
- Fleige E, Quadir M, Haag R. Stimuli-Responsive Polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv Drug Deliver Rev. 2012;64:866–84. - PubMed
-
- Park K, Lee S, Kang E, Kim K, Choi K, Kwon IC. New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv Funct Mater. 2009;19:1553–66.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

