In vivo response to dynamic hyaluronic acid hydrogels
- PMID: 23523533
- PMCID: PMC3674107
- DOI: 10.1016/j.actbio.2013.03.019
In vivo response to dynamic hyaluronic acid hydrogels
Abstract
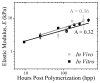
Tissue-specific elasticity arises in part from developmental changes in extracellular matrix over time, e.g. ~10-fold myocardial stiffening in the chicken embryo. When this time-dependent stiffening has been mimicked in vitro with thiolated hyaluronic acid (HA-SH) hydrogels, improved cardiomyocyte maturation has been observed. However, host interactions, matrix polymerization, and the stiffening kinetics remain uncertain in vivo, and each plays a critical role in therapeutic applications using HA-SH. Hematological and histological analysis of subcutaneously injected HA-SH hydrogels showed minimal systemic immune response and host cell infiltration. Most importantly, subcutaneously injected HA-SH hydrogels exhibited time-dependent porosity and stiffness changes at a rate similar to hydrogels polymerized in vitro. When injected intramyocardially host cells begin to actively degrade HA-SH hydrogels within 1week post-injection, continuing this process while producing matrix to nearly replace the hydrogel within 1month post-injection. While non-thiolated HA did not degrade after injection into the myocardium, it also did not elicit an immune response, unlike HA-SH, where visible granulomas and macrophage infiltration were present 1month post-injection, likely due to reactive thiol groups. Altogether these data suggest that the HA-SH hydrogel responds appropriately in a less vascularized niche and stiffens as had been demonstrated in vitro, but in more vascularized tissues, in vivo applicability appears limited.
Copyright © 2013 Acta Materialia Inc. All rights reserved.
Figures





References
-
- Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol. 2005;204:198–209. - PubMed
-
- Zaari N, Rajagopalan P, Kim SK, Engler AJ, Wong JY. Photopolymerization in microfluidic gradient generators: Microscale control of substrate compliance to manipulate cell response. Advanced Materials. 2004;16:2133–7.
-
- Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol. 2008;295:C1037–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

