Expression and purification of active receptor interacting protein 1 kinase using a baculovirus system
- PMID: 23523699
- PMCID: PMC3653991
- DOI: 10.1016/j.pep.2013.03.002
Expression and purification of active receptor interacting protein 1 kinase using a baculovirus system
Abstract
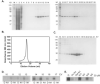
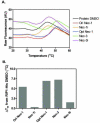
Receptor Interacting Protein 1 (RIP1) kinase is one of the key mediators of tumor necrosis factor alpha (TNF-α) signaling and is critical for activation of necroptotic cell death. We developed a method for expression of recombinant kinase, utilizing baculovirus co-infection of Cdc37, an Hsp90 co-chaperone, and RIP1-His, followed by a two-step purification scheme. After optimization, 1-3mg of highly purified RIP1 kinase was typically obtained from a 1L of Sf9 cells. The recombinant protein displayed kinase activity that was blocked by RIP1 inhibitors, necrostatins. The purified protein was used to develop a simple and robust thermal shift assay for further assessment of RIP1 inhibitors.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Fluorescence polarization assay for inhibitors of the kinase domain of receptor interacting protein 1.Anal Biochem. 2012 Aug 15;427(2):164-74. doi: 10.1016/j.ab.2012.05.019. Epub 2012 May 29. Anal Biochem. 2012. PMID: 22658960 Free PMC article.
-
Structural basis of RIP1 inhibition by necrostatins.Structure. 2013 Mar 5;21(3):493-9. doi: 10.1016/j.str.2013.01.016. Structure. 2013. PMID: 23473668
-
Activity assays for receptor-interacting protein kinase 1:a key regulator of necroptosis.Methods Mol Biol. 2013;1004:31-42. doi: 10.1007/978-1-62703-383-1_3. Methods Mol Biol. 2013. PMID: 23733567
-
Necroptosis: an emerging form of programmed cell death.Crit Rev Oncol Hematol. 2012 Jun;82(3):249-58. doi: 10.1016/j.critrevonc.2011.08.004. Epub 2011 Oct 1. Crit Rev Oncol Hematol. 2012. PMID: 21962882 Review.
-
Inhibitors of RIP1 kinase: a patent review (2016-present).Expert Opin Ther Pat. 2021 Feb;31(2):137-151. doi: 10.1080/13543776.2021.1854729. Epub 2020 Dec 4. Expert Opin Ther Pat. 2021. PMID: 33249869 Review.
Cited by
-
Evaluation of RIP1K and RIP3K expressions in the malignant and benign breast tumors.Tumour Biol. 2016 Jul;37(7):8849-56. doi: 10.1007/s13277-015-4762-7. Epub 2016 Jan 9. Tumour Biol. 2016. PMID: 26749282
-
Structure guided design of potent and selective ponatinib-based hybrid inhibitors for RIPK1.Cell Rep. 2015 Mar 24;10(11):1850-60. doi: 10.1016/j.celrep.2015.02.052. Cell Rep. 2015. PMID: 25801024 Free PMC article.
-
A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis.Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):5017-22. doi: 10.1073/pnas.1505244112. Epub 2015 Apr 7. Proc Natl Acad Sci U S A. 2015. PMID: 25852146 Free PMC article.
References
-
- Dunai Z, Bauer PI, Mihalik R. Necroptosis: Biochemical, Physiological and Pathological Aspects, Pathol. Oncol. Res. 2011;17:791–800. - PubMed
-
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

