A structure-activity relationship study on multi-heterocyclic molecules: two linked thiazoles are required for cytotoxic activity
- PMID: 23524379
- PMCID: PMC3601786
- DOI: 10.1039/C2MD20291C
A structure-activity relationship study on multi-heterocyclic molecules: two linked thiazoles are required for cytotoxic activity
Abstract
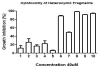
We report the synthesis, cytotoxicity, and phenotypic analysis of oxazole and thiazole containing fragments. Evaluating the optimal size and heterocycle for growth inhibition and apoptosis showed that activity required at least two thiazoles sequentially connected. This is the first detailed comparison of biological activity between multi-heterocyclic containing fragments.
Figures

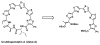
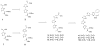


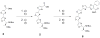

References
-
- Baumann M, Bazendale IR, Ley SV, Nikbin N. Beilstein J Org Chem. 2011;7:422–495. - PMC - PubMed
- Boger DL. Patent application: PCT/US2007/019471/ US2010/0075931 A1. 2012;12:310, 474.
- Dang Q, Yan L, Cashion DK, Kasibhatla SR, Jiang T, Taplin F, Jacintho JD, Li H, Sun Z, Fan Y, DaRe J, Tian F, Li W, Gibson T, Lemus R, van Poelje PD, Potter SC, Erion MD. J Med Chem. 2011;54:153–165. - PubMed
- Dosa PI, Ruble JC, Fu GC. J Org Chem. 1997;62:444–445. - PubMed
- Duchler M. J Drug Target. 2012;20:389–400. - PubMed
- Hughes RA, Moody CJ. Angewandte Chemie International Edition. 2007;46:7930–7954. - PubMed
- Wagner B, Schumann D, Linne U, Koert U, Marahiel MA. J Am Chem Soc. 2006;128:10513–10520. - PubMed
- Sivonen K, Leikoski N, Fewer DP, Jokela J. Appl Microbiol Biotechnol. 2012;86:1213–1225. - PMC - PubMed
-
- Smith RA, Barbosa J, Blum CL, Bobko MA, Caringal YV, Dally R, Johnson JS, Katz ME, Kennure N, Kingery-Wood J, Lee W, Lowinger TB, Lyons J, Marsh V, Rogers DH, Swartz S, Walling T, Wild H. Bioorg Med Chem Lett. 2001;11:2775–2778. - PubMed
- Davis MR, Singh EK, Wahyudi H, Alexander LD, Kunicki J, Nazarova LA, Fairweather KA, Giltrap AM, Jolliffe KA, McAlpine SR. Tetrahedron. 2012;68:1029–1051. - PMC - PubMed
- Singh E, Ramsey DM, McAlpine SR. Org Lett. 2012;14:1198–1201. - PubMed
-
- Biron E, Chatterjee J, Kessler H. Organic Letters. 2006;8:2417–2420. - PubMed
-
- Haberhauer G, Drosdow E, Oeser T, Rominger F. Tetrahedron. 2008;64:1853–1859.
-
- Ma M, Cheng Y, Xu Z, Xu P, Qu H, Fang Y, Xu T, Wen L. European Journal of Medicinal Chemistry. 2007;42:93–98. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

