Allocryptopine and benzyltetrahydropalmatine block hERG potassium channels expressed in HEK293 cells
- PMID: 23524574
- PMCID: PMC4002887
- DOI: 10.1038/aps.2012.176
Allocryptopine and benzyltetrahydropalmatine block hERG potassium channels expressed in HEK293 cells
Abstract
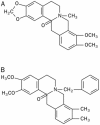
Aim: Allocryptopine (ALL) is an alkaloid extracted from Corydalis decumbens (Thunb) Pers. Papaveraceae, whereas benzyltetrahydropalmatine (BTHP) is a derivative of tetrahydropalmatine extracted from Corydalis ambigua (Pall) Cham et Schlecht. The aim of this study was to investigate the effects of ALL and BTHP on the human ether-a-go-go related gene (hERG) current expressed in HEK293 cells.
Methods: Cultured HEK293 cells were transiently transfected with hERG channel cDNA plasmid pcDNA3.1 using Lipofectamine. The whole-cell current IHERG was evoked and recorded using Axon MultiClamp 700B amplifier. The drugs were applied via supserfusion.
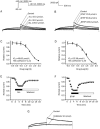
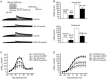
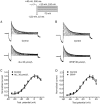
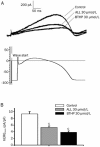
Results: Both ALL and BTHP reversibly suppressed the amplitude and density of IHERG in concentration- and voltage-dependent manners (the respective IC50 value was 49.65 and 22.38 μmol/L). BTHP (30 μmol/L) caused a significant negative shift of the steady-state inactivation curve of IHERG, while ALL (30 μmol/L) did not affect the steady-state inactivation of IHERG. Furthermore, BTHP, but not ALL, shortened the time constants of fast inactivation and slow time constants of deactivation of IHERG. But both the drugs markedly lengthened the time constants for recovery of IHERG from inactivation. Using action potential waveform pulses, it was found that both the drugs at 30 μmol/L significantly suppressed the current densities in the late phase of action potential, but did not significantly affect the current densities in the early phase of action potential.
Conclusion: Both ALL and BTHP derived from Chinese herbs potently block hERG current.
Figures




Similar articles
-
[Effects of allitridum on rapidly delayed rectifier potassium current in HEK293 cell line].Nan Fang Yi Ke Da Xue Xue Bao. 2015 Aug;35(8):1128-32, 1142. Nan Fang Yi Ke Da Xue Xue Bao. 2015. PMID: 26277508 Chinese.
-
Cyclovirobuxine D inhibits the currents of HERG potassium channels stably expressed in HEK293 cells.Eur J Pharmacol. 2011 Jun 25;660(2-3):259-67. doi: 10.1016/j.ejphar.2011.03.039. Epub 2011 Apr 12. Eur J Pharmacol. 2011. PMID: 21497594
-
Impact of the whole-cell patch-clamp configuration on the pharmacological assessment of the hERG channel: trazodone as a case example.J Pharmacol Toxicol Methods. 2014 May-Jun;69(3):237-44. doi: 10.1016/j.vascn.2013.12.007. Epub 2014 Jan 9. J Pharmacol Toxicol Methods. 2014. PMID: 24412489
-
Inhibitory effects of a bisbenzylisoquinline alkaloid dauricine on HERG potassium channels.J Ethnopharmacol. 2012 Jun 1;141(2):685-91. doi: 10.1016/j.jep.2011.08.054. Epub 2011 Sep 5. J Ethnopharmacol. 2012. PMID: 21920426
-
Clemastine, a conventional antihistamine, is a high potency inhibitor of the HERG K+ channel.J Mol Cell Cardiol. 2006 Jan;40(1):107-18. doi: 10.1016/j.yjmcc.2005.09.017. Epub 2005 Nov 9. J Mol Cell Cardiol. 2006. PMID: 16288909
Cited by
-
Natural products modulating the hERG channel: heartaches and hope.Nat Prod Rep. 2017 Aug 2;34(8):957-980. doi: 10.1039/c7np00014f. Nat Prod Rep. 2017. PMID: 28497823 Free PMC article. Review.
-
Shuyu Capsules Relieve Premenstrual Syndrome Depression by Reducing 5-HT3AR and 5-HT3BR Expression in the Rat Brain.Neural Plast. 2016;2016:7950781. doi: 10.1155/2016/7950781. Epub 2016 Sep 20. Neural Plast. 2016. PMID: 27725889 Free PMC article.
-
Computational Evaluation of N-Based Transannular Interactions in Some Model Fused Medium-Sized Heterocyclic Systems and Implications for Drug Design.Molecules. 2023 Feb 8;28(4):1631. doi: 10.3390/molecules28041631. Molecules. 2023. PMID: 36838625 Free PMC article.
-
Novel Bayesian classification models for predicting compounds blocking hERG potassium channels.Acta Pharmacol Sin. 2014 Aug;35(8):1093-102. doi: 10.1038/aps.2014.35. Epub 2014 Jun 30. Acta Pharmacol Sin. 2014. PMID: 24976154 Free PMC article.
-
Exploring the Anti-inflammatory Effects of Protopine Total Alkaloids of Macleaya Cordata (Willd.) R. Br.Front Vet Sci. 2022 Jul 5;9:935201. doi: 10.3389/fvets.2022.935201. eCollection 2022. Front Vet Sci. 2022. PMID: 35865876 Free PMC article.
References
-
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: hERG encodes the IKr potassium channel. Cell. 1995;81:299–307. - PubMed
-
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. hERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–5. - PubMed
-
- Royer A, Demolombe S, El Harchi A, Le Quang K, Piron J, Toumaniantz G, et al. Expression of human ERGK+ channels in the mouse heart exerts anti-arrhythmic activity. Cardiovasc Res. 2005;65:128–37. - PubMed
-
- Spector PS, Curran ME, Keating MT, Sanguinetti MC. Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ Res. 1996;78:499–503. - PubMed
-
- Kiehn J, Thomas D, Karle CA, Schöls W, Kübler W. Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. Naunyn-Schmiedeberg's Arch Pharmacol. 1999;359:212–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

