Generation of cancer stem-like cells through the formation of polyploid giant cancer cells
- PMID: 23524583
- PMCID: PMC3844126
- DOI: 10.1038/onc.2013.96
Generation of cancer stem-like cells through the formation of polyploid giant cancer cells
Erratum in
- Oncogene. 2014 Jan 2;33(1):134
Abstract
Polyploid giant cancer cells (PGCCs) have been observed by pathologists for over a century. PGCCs contribute to solid tumor heterogeneity, but their functions are largely undefined. Little attention has been given to these cells, largely because PGCCs have been generally thought to originate from repeated failure of mitosis/cytokinesis and have no capacity for long-term survival or proliferation. Here we report our successful purification and culture of PGCCs from human ovarian cancer cell lines and primary ovarian cancer. These cells are highly resistant to oxygen deprivation and could form through endoreduplication or cell fusion, generating regular-sized cancer cells quickly through budding or bursting similar to simple organisms like fungi. They express normal and cancer stem cell markers, they divide asymmetrically and they cycle slowly. They can differentiate into adipose, cartilage and bone. A single PGCC formed cancer spheroids in vitro and generated tumors in immunodeficient mice. These PGCC-derived tumors gained a mesenchymal phenotype with increased expression of cancer stem cell markers CD44 and CD133 and become more resistant to treatment with cisplatin. Taken together, our results reveal that PGCCs represent a resistant form of human cancer using an ancient, evolutionarily conserved mechanism in response to hypoxia stress; they can contribute to the generation of cancer stem-like cells, and also play a fundamental role in regulating tumor heterogeneity, tumor growth and chemoresistance in human cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


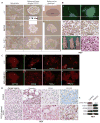
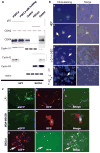

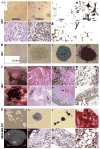

References
-
- Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–2265. - PubMed
-
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. - PubMed
-
- Kumar V, Abbas AL, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of of Disease. 8. 2010. pp. 262–270.
-
- Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. - PubMed
-
- Geigl JB, Obenauf AC, Schwarzbraun T, Speicher MR. Defining ‘chromosomal instability’. Trends Genet. 2008;24:64–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

