Efficacy of an adapted granzyme B-based anti-CD30 cytolytic fusion protein against PI-9-positive classical Hodgkin lymphoma cells in a murine model
- PMID: 23524591
- PMCID: PMC3615217
- DOI: 10.1038/bcj.2013.4
Efficacy of an adapted granzyme B-based anti-CD30 cytolytic fusion protein against PI-9-positive classical Hodgkin lymphoma cells in a murine model
Abstract
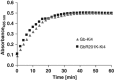
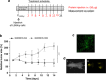
Tumors develop when infiltrating immune cells contribute growth stimuli, and cancer cells are selected to survive within such a cytotoxic microenvironment. One possible immune-escape mechanism is the upregulation of PI-9 (Serpin B9) within cancer cells. This serine proteinase inhibitor selectively inactivates apoptosis-inducing granzyme B (GrB) from cytotoxic granules of innate immune cells. We demonstrate that most classical Hodgkin lymphoma (cHL)-derived cell lines express PI-9, which protects them against the GrB attack and thereby renders them resistant against GrB-based immunotherapeutics. To circumvent this disadvantage, we developed PI-9-insensitive human GrB mutants as fusion proteins to target the Hodgkin-selective receptor CD30. In contrast to the wild-type GrB, a R201K point-mutated GrB construct most efficiently killed PI-9-positive and -negative cHL cells. This was tested in vitro and also in vivo whereby a novel optical imaging-based tumor model with HL cell line L428 was applied. Therefore, this variant, as part of the next generation immunotherapeutics, also named cytolytic fusion proteins showing reduced immunogenicity, is a promising molecule for (targeted) therapy of patients with relapsing malignancies, such as cHL, and possibly other PI-9-positive malignancies, such as breast or lung carcinoma.
Figures




References
-
- Kurschus FC, Jenne DE. Delivery and therapeutic potential of human granzyme B. Immunol Rev. 2010;235:159–171. - PubMed
-
- Liu Y, Cheung LH, Hittelman WN, Rosenblum MG. Targeted delivery of human pro-apoptotic enzymes to tumor cells: in vitro studies describing a novel class of recombinant highly cytotoxic agents. Mol Cancer Ther. 2003;2:1341–1350. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

