Sudden unexpected death in a mouse model of Dravet syndrome
- PMID: 23524966
- PMCID: PMC3613924
- DOI: 10.1172/JCI66220
Sudden unexpected death in a mouse model of Dravet syndrome
Abstract
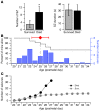
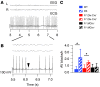
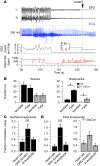
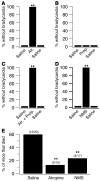
Sudden unexpected death in epilepsy (SUDEP) is the most common cause of death in intractable epilepsies, but physiological mechanisms that lead to SUDEP are unknown. Dravet syndrome (DS) is an infantile-onset intractable epilepsy caused by heterozygous loss-of-function mutations in the SCN1A gene, which encodes brain type-I voltage-gated sodium channel NaV1.1. We studied the mechanism of premature death in Scn1a heterozygous KO mice and conditional brain- and cardiac-specific KOs. Video monitoring demonstrated that SUDEP occurred immediately following generalized tonic-clonic seizures. A history of multiple seizures was a strong risk factor for SUDEP. Combined video-electroencephalography-electrocardiography revealed suppressed interictal resting heart-rate variability and episodes of ictal bradycardia associated with the tonic phases of generalized tonic-clonic seizures. Prolonged atropine-sensitive ictal bradycardia preceded SUDEP. Similar studies in conditional KO mice demonstrated that brain, but not cardiac, KO of Scn1a produced cardiac and SUDEP phenotypes similar to those found in DS mice. Atropine or N-methyl scopolamine treatment reduced the incidence of ictal bradycardia and SUDEP in DS mice. These findings suggest that SUDEP is caused by apparent parasympathetic hyperactivity immediately following tonic-clonic seizures in DS mice, which leads to lethal bradycardia and electrical dysfunction of the ventricle. These results have important implications for prevention of SUDEP in DS patients.
Figures


Comment in
-
Sudden death in epilepsy: of mice and men.J Clin Invest. 2013 Apr;123(4):1415-6. doi: 10.1172/JCI67759. Epub 2013 Mar 25. J Clin Invest. 2013. PMID: 23524959 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

