Novel nanosomes for gene delivery to Plasmodium falciparum-infected red blood cells
- PMID: 23525038
- PMCID: PMC3607119
- DOI: 10.1038/srep01534
Novel nanosomes for gene delivery to Plasmodium falciparum-infected red blood cells
Abstract
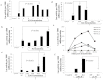
Malaria threatens millions of people annually and is a burden to human health and economic development. Unfortunately in terms of disease control, no effective vaccines are available and the efficacy of treatment is limited by drug resistance. Genetic manipulation in Plasmodium falciparum is hampered due to the absence of robust methods for genetic analyses. Electroporation-based transfection methods have allowed the study of gene function in P. falciparum, with low efficiency. A lipid nanoparticle was developed that allowed nuclear targeting of pDNA with increased efficiency in reporter assay, compared to traditional electroporation method. This method has for the first time, facilitated transfection using both circular and linear DNA in P. falciparum thereby serving as an alternative to electroporation with an increase in transfection efficiency. Availability of a robust method for functional genomic studies in these organisms may be a catalyst for discovery of novel targets for developing drugs and vaccines.
Figures
Similar articles
-
Optimized plasmid loading of human erythrocytes for Plasmodium falciparum DNA transfections.Int J Parasitol. 2024 Oct;54(12):597-605. doi: 10.1016/j.ijpara.2024.04.011. Epub 2024 May 6. Int J Parasitol. 2024. PMID: 38719176
-
Lyse-Reseal Erythrocytes for Transfection of Plasmodium falciparum.Sci Rep. 2019 Dec 27;9(1):19952. doi: 10.1038/s41598-019-56513-9. Sci Rep. 2019. PMID: 31882761 Free PMC article.
-
Comparison of Plasmodium falciparum transfection methods.Malar J. 2003 Jun 27;2:19. doi: 10.1186/1475-2875-2-19. Epub 2003 Jun 27. Malar J. 2003. PMID: 12869208 Free PMC article.
-
Illuminating Plasmodium falciparum-infected red blood cells.Trends Parasitol. 2007 Jun;23(6):268-77. doi: 10.1016/j.pt.2007.04.001. Epub 2007 Apr 16. Trends Parasitol. 2007. PMID: 17434344 Review.
-
Malaria transfection and transfection vectors.Trends Parasitol. 2003 Sep;19(9):381-3. doi: 10.1016/s1471-4922(03)00187-9. Trends Parasitol. 2003. PMID: 12957510 Review.
Cited by
-
Emancipating Chlamydia: Advances in the Genetic Manipulation of a Recalcitrant Intracellular Pathogen.Microbiol Mol Biol Rev. 2016 Mar 30;80(2):411-27. doi: 10.1128/MMBR.00071-15. Print 2016 Jun. Microbiol Mol Biol Rev. 2016. PMID: 27030552 Free PMC article. Review.
-
Nanotechnology Platform for Advancing Vaccine Development against the COVID-19 Virus.Diseases. 2023 Dec 10;11(4):177. doi: 10.3390/diseases11040177. Diseases. 2023. PMID: 38131983 Free PMC article. Review.
-
DNA repair mechanisms and their biological roles in the malaria parasite Plasmodium falciparum.Microbiol Mol Biol Rev. 2014 Sep;78(3):469-86. doi: 10.1128/MMBR.00059-13. Microbiol Mol Biol Rev. 2014. PMID: 25184562 Free PMC article. Review.
-
Optimized plasmid loading of human erythrocytes for Plasmodium falciparum DNA transfections.Int J Parasitol. 2024 Oct;54(12):597-605. doi: 10.1016/j.ijpara.2024.04.011. Epub 2024 May 6. Int J Parasitol. 2024. PMID: 38719176
-
Lyse-Reseal Erythrocytes for Transfection of Plasmodium falciparum.Sci Rep. 2019 Dec 27;9(1):19952. doi: 10.1038/s41598-019-56513-9. Sci Rep. 2019. PMID: 31882761 Free PMC article.
References
-
- Mamoun C. B. et al. Transfer of genes into Plasmodium falciparum by polyamidoamine dendrimers. Mol Biochem Parasitol 103, 117–121 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

