The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory
- PMID: 23525042
- PMCID: PMC3777648
- DOI: 10.1038/nn.3359
The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory
Abstract
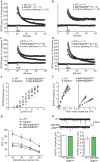
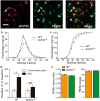
Recent exome sequencing studies have implicated polymorphic Brg1-associated factor (BAF) complexes (mammalian SWI/SNF chromatin remodeling complexes) in several human intellectual disabilities and cognitive disorders. However, it is currently unknown how mutations in BAF complexes result in impaired cognitive function. Postmitotic neurons express a neuron-specific assembly, nBAF, characterized by the neuron-specific subunit BAF53b. Mice harboring selective genetic manipulations of BAF53b have severe defects in long-term memory and long-lasting forms of hippocampal synaptic plasticity. We rescued memory impairments in BAF53b mutant mice by reintroducing BAF53b in the adult hippocampus, which suggests a role for BAF53b beyond neuronal development. The defects in BAF53b mutant mice appeared to derive from alterations in gene expression that produce abnormal postsynaptic components, such as spine structure and function, and ultimately lead to deficits in synaptic plasticity. Our results provide new insight into the role of dominant mutations in subunits of BAF complexes in human intellectual and cognitive disorders.
Figures





References
-
- Zahir FR, Brown CJ. Epigenetic impacts on neurodevelopment: pathophysiological mechanisms and genetic modes of action. Pediatr Res. 2011;69:92R–100R. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 MH073136/MH/NIMH NIH HHS/United States
- MH73136/MH/NIMH NIH HHS/United States
- P01 NS045260/NS/NINDS NIH HHS/United States
- R01 DA025922/DA/NIDA NIH HHS/United States
- R01 DA036984/DA/NIDA NIH HHS/United States
- T32 AG000096/AG/NIA NIH HHS/United States
- T15 LM07443/LM/NLM NIH HHS/United States
- NS 28912/NS/NINDS NIH HHS/United States
- T32-AG00096-29/AG/NIA NIH HHS/United States
- R01 NS028912/NS/NINDS NIH HHS/United States
- DA025922/DA/NIDA NIH HHS/United States
- F31 MH098565/MH/NIMH NIH HHS/United States
- R01 MH081004/MH/NIMH NIH HHS/United States
- R01 NS046789/NS/NINDS NIH HHS/United States
- R01 LM010235/LM/NLM NIH HHS/United States
- R37 NS046789/NS/NINDS NIH HHS/United States
- MH081004/MH/NIMH NIH HHS/United States
- R01 MH101491/MH/NIMH NIH HHS/United States
- LM010235/LM/NLM NIH HHS/United States
- T15 LM007443/LM/NLM NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

