Crystal structure and computational characterization of the lytic polysaccharide monooxygenase GH61D from the Basidiomycota fungus Phanerochaete chrysosporium
- PMID: 23525113
- PMCID: PMC3642327
- DOI: 10.1074/jbc.M113.459396
Crystal structure and computational characterization of the lytic polysaccharide monooxygenase GH61D from the Basidiomycota fungus Phanerochaete chrysosporium
Abstract
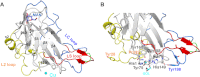
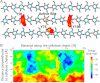
Carbohydrate structures are modified and degraded in the biosphere by a myriad of mostly hydrolytic enzymes. Recently, lytic polysaccharide mono-oxygenases (LPMOs) were discovered as a new class of enzymes for cleavage of recalcitrant polysaccharides that instead employ an oxidative mechanism. LPMOs employ copper as the catalytic metal and are dependent on oxygen and reducing agents for activity. LPMOs are found in many fungi and bacteria, but to date no basidiomycete LPMO has been structurally characterized. Here we present the three-dimensional crystal structure of the basidiomycete Phanerochaete chrysosporium GH61D LPMO, and, for the first time, measure the product distribution of LPMO action on a lignocellulosic substrate. The structure reveals a copper-bound active site common to LPMOs, a collection of aromatic and polar residues near the binding surface that may be responsible for regio-selectivity, and substantial differences in loop structures near the binding face compared with other LPMO structures. The activity assays indicate that this LPMO primarily produces aldonic acids. Last, molecular simulations reveal conformational changes, including the binding of several regions to the cellulose surface, leading to alignment of three tyrosine residues on the binding face of the enzyme with individual cellulose chains, similar to what has been observed for family 1 carbohydrate-binding modules. A calculated potential energy surface for surface translation indicates that P. chrysosporium GH61D exhibits energy wells whose spacing seems adapted to the spacing of cellobiose units along a cellulose chain.
Keywords: Biofuel; CBM33; Carbohydrate-binding Protein; Copper Monooxygenase; GH61; Glycoside Hydrolases; Lytic Polysaccharide Monooxygenase; Molecular Dynamics; Phanerochaete chrysosporium; Structural Biology.
Figures





References
-
- Vaaje-Kolstad G., Westereng B., Horn S. J., Liu Z., Zhai H., Sørlie M., Eijsink V. G. (2010) An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330, 219–222 - PubMed
-
- Phillips C. M., Beeson W. T., Cate J. H., Marletta M. A. (2011) Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem. Biol. 6, 1399–1406 - PubMed
-
- Quinlan R. J., Sweeney M. D., Lo Leggio L., Otten H., Poulsen J.-C., Johansen K. S., Krogh K. B., Jørgensen C. I., Tovborg M., Anthonsen A., Tryfona T., Walter C. P., Dupree P., Xu F., Davies G. J., Walton P. H. (2011) Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. 108, 15079–15084 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

