Effect of endoplasmic reticulum (ER) stress inducer thapsigargin on the expression of extracellular-superoxide dismutase in mouse 3T3-L1 adipocytes
- PMID: 23525536
- PMCID: PMC3593125
- DOI: 10.3164/jcbn.12-46
Effect of endoplasmic reticulum (ER) stress inducer thapsigargin on the expression of extracellular-superoxide dismutase in mouse 3T3-L1 adipocytes
Abstract
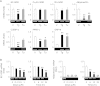
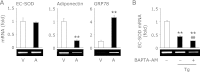
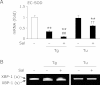
Endoplasmic reticulum stress is related to metabolic disorders, including atherosclerosis and type 2 diabetes. It is known that inflammatory adipocytokines and oxidative stress are increased, while anti-inflammatory adipocytokines such as adiponectin are decreased in adipocytes during above conditions. Extracellular-superoxide dismutase is an anti-inflammatory enzyme that protects cells from oxidative stress. Because plasma extracellular-superoxide dismutase levels in type 2 diabetes patients were inversely related to the body mass index and homeostasis model assessment-insulin resistance index, it is speculated that the regulation of extracellular-superoxide dismutase might lead to the suppression of metabolic disorders. Here, we observed the reduction of extracellular-superoxide dismutase and adiponectin in 3T3-L1 adipocytes treated with thapsigargin, an endoplasmic reticulum stress inducer. Interestingly, tunicamycin, another endoplasmic reticulum stress inducer, did not decrease the expression of extracellular-superoxide dismutase in spite of the induction of glucose regulated protein kinase 78 kDa, an endoplasmic reticulum stress marker. Moreover, eukaryotic translation initiation factor 2α signaling cascade plays a pivotal role in the reduction of extracellular-superoxide dismutase in 3T3-L1 adipocytes during endoplasmic reticulum stress conditions.
Keywords: endoplasmic reticulum stress; eukaryotic translation initiation factor 2α; extracellular-superoxide dismutase; obesity; reactive oxygen species.
Figures

Similar articles
-
Expression of extracellular superoxide dismutase during adipose differentiation in 3T3-L1 cells.Redox Rep. 2009;14(1):34-40. doi: 10.1179/135100009X392467. Redox Rep. 2009. PMID: 19161676
-
The effect of hypoxia mimetic cobalt chloride on the expression of EC-SOD in 3T3-L1 adipocytes.Redox Rep. 2010;15(3):131-7. doi: 10.1179/174329210X12650506623483. Redox Rep. 2010. PMID: 20594416 Free PMC article.
-
Cytoprotective role of the fatty acid binding protein 4 against oxidative and endoplasmic reticulum stress in 3T3-L1 adipocytes.FEBS Open Bio. 2014 Jul 3;4:602-10. doi: 10.1016/j.fob.2014.06.008. eCollection 2014. FEBS Open Bio. 2014. PMID: 25161868 Free PMC article.
-
Endoplasmic reticulum (ER) localization is critical for DsbA-L protein to suppress ER stress and adiponectin down-regulation in adipocytes.J Biol Chem. 2015 Apr 17;290(16):10143-8. doi: 10.1074/jbc.M115.645416. Epub 2015 Mar 4. J Biol Chem. 2015. PMID: 25739441 Free PMC article.
-
ER stress inducer, thapsigargin, decreases extracellular-superoxide dismutase through MEK/ERK signalling cascades in COS7 cells.Free Radic Res. 2011 Jun;45(6):692-8. doi: 10.3109/10715762.2011.567985. Epub 2011 Mar 21. Free Radic Res. 2011. PMID: 21417786
Cited by
-
Interplay between Endoplasmic Reticulum Stress and Large Extracellular Vesicles (Microparticles) in Endothelial Cell Dysfunction.Biomedicines. 2020 Oct 12;8(10):409. doi: 10.3390/biomedicines8100409. Biomedicines. 2020. PMID: 33053883 Free PMC article. Review.
-
Orthogonally-tunable and ER-targeting fluorophores detect avian influenza virus early infection.Nat Commun. 2022 Oct 4;13(1):5841. doi: 10.1038/s41467-022-33586-1. Nat Commun. 2022. PMID: 36192426 Free PMC article.
-
Autophagy modulators in type 2 diabetes: A new perspective.J Diabetes. 2024 Dec;16(12):e70010. doi: 10.1111/1753-0407.70010. J Diabetes. 2024. PMID: 39676616 Free PMC article. Review.
-
Inhibition of protein phosphatase 1 stimulates noncanonical ER stress eIF2α activation to enhance fisetin-induced chemosensitivity in HDAC inhibitor-resistant hepatocellular carcinoma cells.Cancers (Basel). 2019 Jun 29;11(7):918. doi: 10.3390/cancers11070918. Cancers (Basel). 2019. PMID: 31261976 Free PMC article.
-
Calreticulin nuclear translocalization alleviates CaM/CaMKII/CREB signaling pathway to enhance chemosensitivity in HDAC inhibitor-resistant hepatocellular carcinoma cells.Aging (Albany NY). 2022 Jun 20;14(12):5097-5115. doi: 10.18632/aging.204131. Epub 2022 Jun 20. Aging (Albany NY). 2022. PMID: 35724265 Free PMC article.
References
-
- Eriksson JW. Metabolic stress in insulin’s target cells leads to ROS accumulation—a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581:3734–3742. - PubMed
-
- Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. - PubMed
-
- Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. - PubMed
-
- Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. - PubMed
-
- Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

