Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease
- PMID: 23525573
- PMCID: PMC3963538
- DOI: 10.1136/gutjnl-2012-303252
Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease
Abstract
Objective: Proline-glycine-proline (PGP) has been shown to have chemotactic effects on neutrophils via CXCR2 in several lung diseases. PGP is derived from collagen by the combined action of matrix metalloproteinase (MMP) 8 and/or MMP9 and prolyl endopeptidase (PE). We investigated the role of PGP in inflammatory bowel disease (IBD).
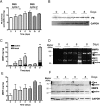
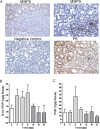
Design: In intestinal tissue from patients with IBD and mice with dextran sodium sulfate (DSS)-induced colitis, MMP8, MMP9 and PE were evaluated by ELISA, immunoblot and immunohistochemistry. Peripheral blood polymorphonuclear cell (PMN) supernatants were also analysed accordingly and incubated with collagen to assess PGP generation ex vivo. PGP levels were measured by mass spectrometry, and PGP neutralisation was achieved with a PGP antagonist and PGP antibodies.
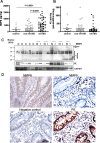
Results: In the intestine of patients with IBD, MMP8 and MMP9 levels were elevated, while PE was expressed at similar levels to control tissue. PGP levels were increased in intestinal tissue of patients with IBD. Similar results were obtained in intestine from DSS-treated mice. PMN supernatants from patients with IBD were far more capable of generating PGP from collagen ex vivo than healthy controls. Furthermore, PGP neutralisation during DSS-induced colitis led to a significant reduction in neutrophil infiltration in the intestine.
Conclusions: The proteolytic cascade that generates PGP from collagen, as well as the tripeptide itself, is present in the intestine of patients with IBD and mice with DSS-induced colitis. PGP neutralisation in DSS-treated mice showed the importance of PGP-guided neutrophilic infiltration in the intestine and indicates a vicious circle in neutrophilic inflammation in IBD.
Figures

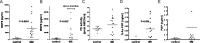

Similar articles
-
Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown.Am J Physiol Lung Cell Mol Physiol. 2011 Feb;300(2):L255-65. doi: 10.1152/ajplung.00304.2010. Epub 2010 Nov 26. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21112944 Free PMC article.
-
Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation?PLoS One. 2013;8(1):e55612. doi: 10.1371/journal.pone.0055612. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383243 Free PMC article.
-
A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation.J Immunol. 2008 Apr 15;180(8):5662-9. doi: 10.4049/jimmunol.180.8.5662. J Immunol. 2008. PMID: 18390751 Free PMC article.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease.Inflamm Bowel Dis. 2013 Jun;19(7):1556-65. doi: 10.1097/MIB.0b013e318281f54e. Inflamm Bowel Dis. 2013. PMID: 23598816 Free PMC article. Review.
Cited by
-
The development of allergic inflammation in a murine house dust mite asthma model is suppressed by synbiotic mixtures of non-digestible oligosaccharides and Bifidobacterium breve M-16V.Eur J Nutr. 2016 Apr;55(3):1141-51. doi: 10.1007/s00394-015-0928-8. Epub 2015 May 24. Eur J Nutr. 2016. PMID: 26003185 Free PMC article.
-
NLRP3 activation in neutrophils induces lethal autoinflammation, liver inflammation, and fibrosis.EMBO Rep. 2022 Nov 7;23(11):e54446. doi: 10.15252/embr.202154446. Epub 2022 Oct 4. EMBO Rep. 2022. PMID: 36194627 Free PMC article.
-
An Analysis of the Content of Metalloproteinases in the Intestinal Wall of Patients with Crohn's Disease.Life (Basel). 2023 Oct 5;13(10):2013. doi: 10.3390/life13102013. Life (Basel). 2023. PMID: 37895400 Free PMC article.
-
Dietary black raspberries modulate DNA methylation in dextran sodium sulfate (DSS)-induced ulcerative colitis.Carcinogenesis. 2013 Dec;34(12):2842-50. doi: 10.1093/carcin/bgt310. Epub 2013 Sep 25. Carcinogenesis. 2013. PMID: 24067901 Free PMC article.
-
An Oral Rinse Active Matrix Metalloproteinase-8 Point-of-Care Immunotest May Be Less Accurate in Patients with Crohn's Disease.Biomolecules. 2020 Mar 4;10(3):395. doi: 10.3390/biom10030395. Biomolecules. 2020. PMID: 32143418 Free PMC article. Clinical Trial.
References
-
- Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. The N Engl J Med 1998;338:436–45 - PubMed
-
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci 2008;13:2400–7 - PubMed
-
- Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Ann Rev Pharmacol Toxicol 2008;48:171–97 - PubMed
-
- Laskin DL, Kimura T, Sakakibara S, et al. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J Leukoc Biol 1986;39:255–66 - PubMed
-
- Weathington NM, van Houwelingen AH, Noerager BD, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 2006;12:317–23 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
