Quaking and PTB control overlapping splicing regulatory networks during muscle cell differentiation
- PMID: 23525800
- PMCID: PMC3677278
- DOI: 10.1261/rna.038422.113
Quaking and PTB control overlapping splicing regulatory networks during muscle cell differentiation
Abstract
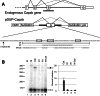
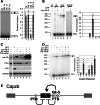
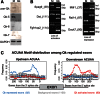
Alternative splicing contributes to muscle development, but a complete set of muscle-splicing factors and their combinatorial interactions are unknown. Previous work identified ACUAA ("STAR" motif) as an enriched intron sequence near muscle-specific alternative exons such as Capzb exon 9. Mass spectrometry of myoblast proteins selected by the Capzb exon 9 intron via RNA affinity chromatography identifies Quaking (QK), a protein known to regulate mRNA function through ACUAA motifs in 3' UTRs. We find that QK promotes inclusion of Capzb exon 9 in opposition to repression by polypyrimidine tract-binding protein (PTB). QK depletion alters inclusion of 406 cassette exons whose adjacent intron sequences are also enriched in ACUAA motifs. During differentiation of myoblasts to myotubes, QK levels increase two- to threefold, suggesting a mechanism for QK-responsive exon regulation. Combined analysis of the PTB- and QK-splicing regulatory networks during myogenesis suggests that 39% of regulated exons are under the control of one or both of these splicing factors. This work provides the first evidence that QK is a global regulator of splicing during muscle development in vertebrates and shows how overlapping splicing regulatory networks contribute to gene expression programs during differentiation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
