Animal models of fibrotic lung disease
- PMID: 23526222
- PMCID: PMC3824038
- DOI: 10.1165/rcmb.2013-0094TR
Animal models of fibrotic lung disease
Abstract
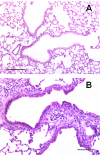
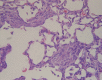
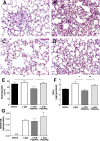
Interstitial lung fibrosis can develop as a consequence of occupational or medical exposure, as a result of genetic defects, and after trauma or acute lung injury leading to fibroproliferative acute respiratory distress syndrome, or it can develop in an idiopathic manner. The pathogenesis of each form of lung fibrosis remains poorly understood. They each result in a progressive loss of lung function with increasing dyspnea, and most forms ultimately result in mortality. To better understand the pathogenesis of lung fibrotic disorders, multiple animal models have been developed. This review summarizes the common and emerging models of lung fibrosis to highlight their usefulness in understanding the cell-cell and soluble mediator interactions that drive fibrotic responses. Recent advances have allowed for the development of models to study targeted injuries of Type II alveolar epithelial cells, fibroblastic autonomous effects, and targeted genetic defects. Repetitive dosing in some models has more closely mimicked the pathology of human fibrotic lung disease. We also have a much better understanding of the fact that the aged lung has increased susceptibility to fibrosis. Each of the models reviewed in this report offers a powerful tool for studying some aspect of fibrotic lung disease.
Figures




References
-
- American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
-
- Coultas D, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung disease. Am J Respir Crit Care Med. 1994;150:967–972. - PubMed
-
- Monaghan H, Wells AU, Colby TV, du Bois RM, Hansell DM, Nicholson AG. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest. 2004;125:522–526. - PubMed
-
- Perez A, Rogers R, Dauber J. The prognosis of idopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S19–S26. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

