JC virus antibody status underestimates infection rates
- PMID: 23526716
- PMCID: PMC3737275
- DOI: 10.1002/ana.23893
JC virus antibody status underestimates infection rates
Abstract
Objective: JC virus (JCV) seropositivity is a risk factor for progressive multifocal leukoencephalopathy (PML) in patients on natalizumab. Accordingly, the JCV serological antibody test is of paramount importance in determining disease risk.
Methods: We tested the accuracy of the JCV serum antibody test by comparing the results of JCV serology to JCV viruria and viremia in 67 patients enrolled in a single-center, retrospective cohort study. Bodily fluids (urine and blood) were assessed for JCV DNA by real time quantitative polymerase chain reaction 6 to 47 months (mean = 26.1 months) before JCV antibody testing. In 10 individuals, blood and urine samples were obtained on 2 separate occasions at 6-month intervals.
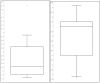
Results: Forty (59.7%) of the 67 patients were JCV seropositive. Of 27 JCV seronegative patients, 10 (37%) had JCV viruria. Urine JCV DNA copy numbers were significantly higher in the seropositive group (mean log copy number = 5.93, range = 1.85-9.21) than the seronegative group (mean log copy number = 2.41, range = 1.85-5.43; p = 0.0026). Considering all body fluid test results, 50 (74.6%) of the 67 patients were previously infected with JCV.
Interpretation: The false-negative rate of the JCV serology in this study was 37%; therefore, JCV serostatus does not appear to identify all patients infected with JCV. Thus, a negative JCV antibody result should not be conflated with absence of JCV infection. This discordance may be important in understanding JCV biology, risk for PML, and PML pathogenesis.
Copyright © 2013 American Neurological Association.
Figures
References
-
- Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74. - PubMed
-
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81. - PubMed
-
- Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353:362–8. - PubMed
-
- [Accessed August 20, 2012];TYSABRI Safety Update. 2012 at https://medinfo.biogenidec.com/medinfo/secure/pmlresource.do?resource=TY....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources