Magnetic resonance imaging is the preferred method to assess treatment-related skeletal changes in children with brain tumors
- PMID: 23526749
- PMCID: PMC4309017
- DOI: 10.1002/pbc.24536
Magnetic resonance imaging is the preferred method to assess treatment-related skeletal changes in children with brain tumors
Abstract
Purpose: To evaluate the growing skeleton for potential altered skeletalgenesis associated with antiangiogenesis therapy.
Patients and methods: Knee radiographs and magnetic resonance imaging (MRI) were prospectively obtained on patients enrolled on two consecutive clinical trials using vandetanib, a potent oral (VEGF receptor 2) VEGFR-2 inhibitor alone or combined with dasatinib, a multiple tyrosine kinase inhibitor, in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG).
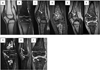
Results: Fifty-nine patients (32 females) underwent 119 MRIs; 51 patients underwent 89 radiographs of the knees. The median age at enrollment was 6.2 years (range, 2.4-17.6 years). The dose of vandetanib ranged from 50 to 145 mg/m(2) /day. The median treatment duration was 205 days. Only two patients have not experienced disease progression after 18 and 60 months from diagnosis. MRI identified clinically significant premature physeal fusion in both knees of one patient, focal physeal thickening in one, osteonecrosis in eight patients (present at enrollment in one), and bony spicules crossing the physis in two patients (bilateral in one). MRI follow-up period averaged 5.3 months (range, 0-25.5 months; median, 3.5 months). Radiographs delineated normally fused physes in two patients but no cases of premature physeal fusion, osteonecrosis or bony spicules.
Conclusions: As MRI provided greater information than radiographs, and thus would be a more sensitive test to assess skeletalgenesis in pediatric patients.
Keywords: VEGF; antiangiogenesis agents; chemotherapy; magnetic resonance imaging; pediatric brain tumors; skeletalgenesis.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Nothing to declare.
Figures
Similar articles
-
Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma.Clin Cancer Res. 2013 Jun 1;19(11):3050-8. doi: 10.1158/1078-0432.CCR-13-0306. Epub 2013 Mar 27. Clin Cancer Res. 2013. PMID: 23536435 Free PMC article. Clinical Trial.
-
Treatment-Related Noncontiguous Radiologic Changes in Children With Diffuse Intrinsic Pontine Glioma Treated With Expanded Irradiation Fields and Antiangiogenic Therapy.Int J Radiat Oncol Biol Phys. 2017 Dec 1;99(5):1295-1305. doi: 10.1016/j.ijrobp.2017.08.021. Epub 2017 Aug 24. Int J Radiat Oncol Biol Phys. 2017. PMID: 29165288 Free PMC article. Clinical Trial.
-
Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma.J Clin Oncol. 2010 Nov 1;28(31):4762-8. doi: 10.1200/JCO.2010.30.3545. Epub 2010 Oct 4. J Clin Oncol. 2010. PMID: 20921456 Free PMC article. Clinical Trial.
-
Dasatinib: in chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia.BioDrugs. 2008;22(1):59-69. doi: 10.2165/00063030-200822010-00007. BioDrugs. 2008. PMID: 18215092 Review.
-
[Management of chronic myeloid leukemia].Rinsho Ketsueki. 2008 Oct;49(10):1401-10. Rinsho Ketsueki. 2008. PMID: 18833925 Review. Japanese. No abstract available.
Cited by
-
Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma.Clin Cancer Res. 2013 Jun 1;19(11):3050-8. doi: 10.1158/1078-0432.CCR-13-0306. Epub 2013 Mar 27. Clin Cancer Res. 2013. PMID: 23536435 Free PMC article. Clinical Trial.
-
Growth plate abnormalities in pediatric cancer patients undergoing phase 1 anti-angiogenic therapy: a report from the Children's Oncology Group Phase I Consortium.Pediatr Blood Cancer. 2015 Jan;62(1):45-51. doi: 10.1002/pbc.25229. Epub 2014 Sep 24. Pediatr Blood Cancer. 2015. PMID: 25257751 Free PMC article. Clinical Trial.
References
-
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. - PubMed
-
- Zondor SD, Medina PJ. Bevacizumab: An angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother. 2004;38:1258–1264. - PubMed
-
- Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A children’s oncology group study. J Clin Oncol. 2008;26:399–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

