Magnetic resonance imaging is the preferred method to assess treatment-related skeletal changes in children with brain tumors
- PMID: 23526749
- PMCID: PMC4309017
- DOI: 10.1002/pbc.24536
Magnetic resonance imaging is the preferred method to assess treatment-related skeletal changes in children with brain tumors
Abstract
Purpose: To evaluate the growing skeleton for potential altered skeletalgenesis associated with antiangiogenesis therapy.
Patients and methods: Knee radiographs and magnetic resonance imaging (MRI) were prospectively obtained on patients enrolled on two consecutive clinical trials using vandetanib, a potent oral (VEGF receptor 2) VEGFR-2 inhibitor alone or combined with dasatinib, a multiple tyrosine kinase inhibitor, in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG).
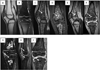
Results: Fifty-nine patients (32 females) underwent 119 MRIs; 51 patients underwent 89 radiographs of the knees. The median age at enrollment was 6.2 years (range, 2.4-17.6 years). The dose of vandetanib ranged from 50 to 145 mg/m(2) /day. The median treatment duration was 205 days. Only two patients have not experienced disease progression after 18 and 60 months from diagnosis. MRI identified clinically significant premature physeal fusion in both knees of one patient, focal physeal thickening in one, osteonecrosis in eight patients (present at enrollment in one), and bony spicules crossing the physis in two patients (bilateral in one). MRI follow-up period averaged 5.3 months (range, 0-25.5 months; median, 3.5 months). Radiographs delineated normally fused physes in two patients but no cases of premature physeal fusion, osteonecrosis or bony spicules.
Conclusions: As MRI provided greater information than radiographs, and thus would be a more sensitive test to assess skeletalgenesis in pediatric patients.
Keywords: VEGF; antiangiogenesis agents; chemotherapy; magnetic resonance imaging; pediatric brain tumors; skeletalgenesis.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Nothing to declare.
Figures
References
-
- Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. - PubMed
-
- Zondor SD, Medina PJ. Bevacizumab: An angiogenesis inhibitor with efficacy in colorectal and other malignancies. Ann Pharmacother. 2004;38:1258–1264. - PubMed
-
- Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: A children’s oncology group study. J Clin Oncol. 2008;26:399–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

