Retroviral and lentiviral vectors for the induction of immunological tolerance
- PMID: 23526794
- PMCID: PMC3605697
- DOI: 10.6064/2012/694137
Retroviral and lentiviral vectors for the induction of immunological tolerance
Abstract
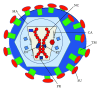
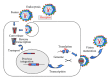
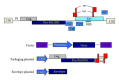
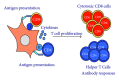
Retroviral and lentiviral vectors have proven to be particularly efficient systems to deliver genes of interest into target cells, either in vivo or in cell cultures. They have been used for some time for gene therapy and the development of gene vaccines. Recently retroviral and lentiviral vectors have been used to generate tolerogenic dendritic cells, key professional antigen presenting cells that regulate immune responses. Thus, three main approaches have been undertaken to induce immunological tolerance; delivery of potent immunosuppressive cytokines and other molecules, modification of intracellular signalling pathways in dendritic cells, and de-targeting transgene expression from dendritic cells using microRNA technology. In this review we briefly describe retroviral and lentiviral vector biology, and their application to induce immunological tolerance.
Keywords: Autoimmune disease; MAPK; NF-κB; T cell; arthritis; co-stimulation; dendritic cell; immunotherapy; microRNA; signalling; tolerance.
Figures
References
-
- Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33(1):153–159. - PubMed
-
- Anderson WF, Blaese RM, Culver K. The ADA Human Gene Therapy clinical protocol: points to consider response with clinical protocol, July 6, 1990. Human Gene Therapy. 1990;1(3):331–362. - PubMed
-
- Blaese RM, Culver K, Culver K, et al. Treatment of severe combined immunodeficiency disease (SCID) due to adenosine deaminase deficiency with autologous lymphocytes transduced with a human ADA gene. Human Gene Therapy. 1993;4(4):521–527. - PubMed
-
- Cavazzana-Calvo M, Thrasher A, Mavillo F. The future of gene therapy. Nature. 2004;427(6977):779–781. - PubMed
-
- Levine F, Friedmann T. Gene therapy techniques. Current Opinion in Biotechnology. 1991;2(6):840–844. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

