Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling
- PMID: 23526820
- PMCID: PMC3633673
- DOI: 10.4049/jimmunol.1202096
Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling
Abstract
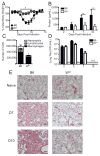
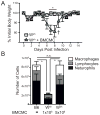
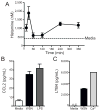
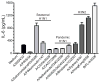
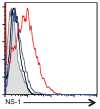
Influenza A virus (IAV) is a major respiratory pathogen of both humans and animals. The lung is protected from pathogens by alveolar epithelial cells, tissue-resident alveolar macrophages, dendritic cells, and mast cells. The role of alveolar epithelial cells, endothelial cells, and alveolar macrophages during IAV infection has been studied previously. In this study, we address the role of mast cells during IAV infection. Respiratory infection with A/WSN/33 causes significant disease and immunopathology in C57BL/6 mice but not in B6.Cg-Kit(W-sh) mice, which lack mast cells. During in vitro coculture, A/WSN/33 caused mast cells to release histamine, secrete cytokines and chemokines, and produce leukotrienes. Moreover, when mast cells were infected with IAV, the virus did not replicate within mast cells. Importantly, human H1N1, H3N2, and influenza B virus isolates also could activate mast cells in vitro. Mast cell production of cytokines and chemokines occurs in a RIG-I/MAVS-dependent mechanism; in contrast, histamine production occurred through a RIG-I/MAVS-independent mechanism. Our data highlight that, following IAV infection, the response of mast cells is controlled by multiple receptors. In conclusion, we identified a unique inflammatory cascade activated during IAV infection that could potentially be targeted to limit morbidity following IAV infection.
Figures

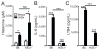
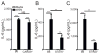
References
-
- Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, Garcia-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci U S A. 2009;106:3455–3460. - PMC - PubMed
-
- Baas T, Baskin CR, Diamond DL, Garcia-Sastre A, Bielefeldt-Ohmann H, Tumpey TM, Thomas MJ, Carter VS, Teal TH, Van Hoeven N, Proll S, Jacobs JM, Caldwell ZR, Gritsenko MA, Hukkanen RR, Camp DG, 2nd, Smith RD, Katze MG. Integrated molecular signature of disease: analysis of influenza virus-infected macaques through functional genomics and proteomics. J Virol. 2006;80:10813–10828. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

