Binding of the 9-O-N-aryl/arylalkyl amino carbonyl methyl substituted berberine analogs to tRNA(phe.)
- PMID: 23526972
- PMCID: PMC3602459
- DOI: 10.1371/journal.pone.0058279
Binding of the 9-O-N-aryl/arylalkyl amino carbonyl methyl substituted berberine analogs to tRNA(phe.)
Abstract
Background: Three new analogs of berberine with aryl/ arylalkyl amino carbonyl methyl substituent at the 9-position of the isoquinoline chromophore along with berberrubine were studied for their binding to tRNA(phe) by wide variety of biophysical techniques like spectrophotometry, spectrofluorimetry, circular dichroism, thermal melting, viscosity and isothermal titration calorimetry.
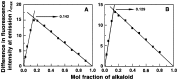
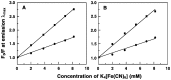
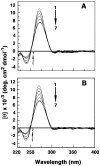
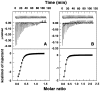
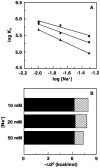
Methodology/ principal findings: Scatchard binding isotherms revealed that the cooperative binding mode of berberine was propagated in the analogs also. Thermal melting studies showed that all the 9-O-N-aryl/arylalkyl amino carbonyl methyl substituted berberine analogs stabilized the tRNA(phe) more in comparison to berberine. Circular dichroism studies showed that these analogs perturbed the structure of tRNA(phe) more in comparison to berberine. Ferrocyanide quenching studies and viscosity results proved the intercalative binding mode of these analogs into the helical organization of tRNA(phe). The binding was entropy driven for the analogs in sharp contrast to the enthalpy driven binding of berberine. The introduction of the aryl/arylalkyl amino carbonyl methyl substituent at the 9-position thus switched the enthalpy driven binding of berberine to entropy dominated binding. Salt and temperature dependent calorimetric studies established the involvement of multiple weak noncovalent interactions in the binding process.
Conclusions/ significance: The results showed that 9-O-N-aryl/arylalkyl amino carbonyl methyl substituted berberine analogs exhibited almost ten folds higher binding affinity to tRNA(phe) compared to berberine whereas the binding of berberrubine was dramatically reduced by about twenty fold in comparison to berberine. The spacer length of the substitution at the 9-position of the isoquinoline chromophore appears to be critical in modulating the binding affinities towards tRNA(phe).
Conflict of interest statement
Figures
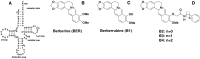

Similar articles
-
Synthesis of novel 9-O-N-aryl/aryl-alkyl amino carbonyl methyl substituted berberine analogs and evaluation of DNA binding aspects.Bioorg Med Chem. 2012 Apr 15;20(8):2498-505. doi: 10.1016/j.bmc.2012.03.006. Epub 2012 Mar 8. Bioorg Med Chem. 2012. PMID: 22459209
-
Photophysical and calorimetric studies on the binding of 9-O-substituted analogs of the plant alkaloid berberine to double stranded poly(A).J Photochem Photobiol B. 2013 Aug 5;125:105-14. doi: 10.1016/j.jphotobiol.2013.05.009. Epub 2013 Jun 4. J Photochem Photobiol B. 2013. PMID: 23792948
-
Binding of novel 9-O-N-aryl/arylalkyl amino carbonyl methyl berberine analogs to poly(U)-poly(A)·poly(U) triplex and comparison to the duplex poly(A)-poly(U).Mol Biol Rep. 2014 Aug;41(8):5473-83. doi: 10.1007/s11033-014-3421-1. Epub 2014 May 30. Mol Biol Rep. 2014. PMID: 24874303
-
Ribosomal binding of modified tRNA anticodons related to thermal stability.Nucleic Acids Symp Ser. 1997;(36):58-60. Nucleic Acids Symp Ser. 1997. PMID: 9478206 Review.
-
Recent Advances in Nucleic Acid Binding Aspects of Berberine Analogs and Implications for Drug Design.Mini Rev Med Chem. 2016;16(2):104-19. doi: 10.2174/1389557515666150909144425. Mini Rev Med Chem. 2016. PMID: 26349491 Review.
Cited by
-
A real-time fluorescence polarization activity assay to screen for inhibitors of bacterial ribonuclease P.Nucleic Acids Res. 2014 Nov 10;42(20):e159. doi: 10.1093/nar/gku850. Epub 2014 Sep 23. Nucleic Acids Res. 2014. PMID: 25249623 Free PMC article.
-
G-quadruplex ligands mediate downregulation of DUX4 expression.Nucleic Acids Res. 2020 May 7;48(8):4179-4194. doi: 10.1093/nar/gkaa146. Nucleic Acids Res. 2020. PMID: 32182342 Free PMC article.
-
Therapeutic Strategies Targeting DUX4 in FSHD.J Clin Med. 2020 Sep 7;9(9):2886. doi: 10.3390/jcm9092886. J Clin Med. 2020. PMID: 32906621 Free PMC article. Review.
-
New substrates and determinants for tRNA recognition of RNA methyltransferase DNMT2/TRDMT1.RNA Biol. 2021 Dec;18(12):2531-2545. doi: 10.1080/15476286.2021.1930756. Epub 2021 Jun 10. RNA Biol. 2021. PMID: 34110975 Free PMC article.
References
-
- Gesteland RF, Cech TR, Atkins JF (Eds.) (2006) The RNA world. New York, NY: Cold Spring Harbor Laboratory Press.
-
- Ambros V (2004) The functions of animal microRNAs. Nature 431: 350–355. - PubMed
-
- Bartel DP (2004) MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281–297. - PubMed
-
- Gait MJ, Karn J (1995) Progress in anti-HIV structure-based drug design. Trends Biotechnol 10: 430–438. - PubMed
-
- Gallego J, Varani G (2001) Targeting RNA with small-molecule drugs: Therapeutic opportunities and chemical challenges. Acc Chem Res 34: 836–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

