Leucine and protein metabolism in obese Zucker rats
- PMID: 23527196
- PMCID: PMC3603883
- DOI: 10.1371/journal.pone.0059443
Leucine and protein metabolism in obese Zucker rats
Abstract
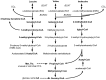
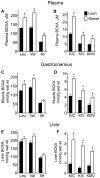
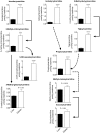
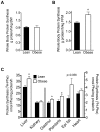
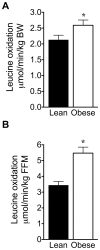
Branched-chain amino acids (BCAAs) are circulating nutrient signals for protein accretion, however, they increase in obesity and elevations appear to be prognostic of diabetes. To understand the mechanisms whereby obesity affects BCAAs and protein metabolism, we employed metabolomics and measured rates of [1-(14)C]-leucine metabolism, tissue-specific protein synthesis and branched-chain keto-acid (BCKA) dehydrogenase complex (BCKDC) activities. Male obese Zucker rats (11-weeks old) had increased body weight (BW, 53%), liver (107%) and fat (∼300%), but lower plantaris and gastrocnemius masses (-21-24%). Plasma BCAAs and BCKAs were elevated 45-69% and ∼100%, respectively, in obese rats. Processes facilitating these rises appeared to include increased dietary intake (23%), leucine (Leu) turnover and proteolysis [35% per g fat free mass (FFM), urinary markers of proteolysis: 3-methylhistidine (183%) and 4-hydroxyproline (766%)] and decreased BCKDC per g kidney, heart, gastrocnemius and liver (-47-66%). A process disposing of circulating BCAAs, protein synthesis, was increased 23-29% by obesity in whole-body (FFM corrected), gastrocnemius and liver. Despite the observed decreases in BCKDC activities per gm tissue, rates of whole-body Leu oxidation in obese rats were 22% and 59% higher normalized to BW and FFM, respectively. Consistently, urinary concentrations of eight BCAA catabolism-derived acylcarnitines were also elevated. The unexpected increase in BCAA oxidation may be due to a substrate effect in liver. Supporting this idea, BCKAs were elevated more in liver (193-418%) than plasma or muscle, and per g losses of hepatic BCKDC activities were completely offset by increased liver mass, in contrast to other tissues. In summary, our results indicate that plasma BCKAs may represent a more sensitive metabolic signature for obesity than BCAAs. Processes supporting elevated BCAA]BCKAs in the obese Zucker rat include increased dietary intake, Leu and protein turnover along with impaired BCKDC activity. Elevated BCAAs/BCKAs may contribute to observed elevations in protein synthesis and BCAA oxidation.
Conflict of interest statement
Figures



References
-
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, et al. (2006) Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930. - PubMed
-
- Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, et al. (2002) Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab 283: E503–513. - PubMed
-
- Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, et al. (2006) Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. American journal of physiology Endocrinology and metabolism 291: E621–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

