Synergistic effect of afatinib with su11274 in non-small cell lung cancer cells resistant to gefitinib or erlotinib
- PMID: 23527257
- PMCID: PMC3601073
- DOI: 10.1371/journal.pone.0059708
Synergistic effect of afatinib with su11274 in non-small cell lung cancer cells resistant to gefitinib or erlotinib
Abstract
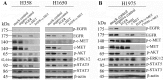
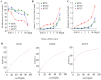
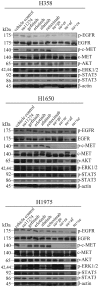
Epidermal growth factor receptor (EGFR) and c-MET receptors are expressed on many non-small cell lung cancer (NSCLC) cells. Current single agent therapeutic targeting of a mutant EGFR has a high efficacy in the clinic, but is not curative. Here, we investigated the combination of targeting EGFR and c-MET pathways in NSCLC cells resistant to receptor tyrosine kinase inhibitors (TKIs), using RNA interference and inhibition by TKIs. Different NSCLC cell lines with various genomic characteristics (H358, H1650 and H1975) were transfected with EGFR-specific-siRNA, T790M-specific-siRNA, c-MET siRNA or the combination. Subsequently EGFR TKIs (gefitinib, erlotinib or afatinib) or monoclonal antibody cetuximab were combined respectively with the c-MET-specific TKI su11274 in NSCLC cell lines. The cell proliferation, viability, caspase-3/7 activity and apoptotic morphology were monitored by spectrophotometry, fluorimetry and fluorescence microscopy. The combined effect of EGFR TKIs, or cetuximab and su11274, was evaluated using a combination index. The results showed that the cell lines that were relatively resistant to EGFR TKIs, especially the H1975 cell line containing the resistance T790M mutation, were found to be more sensitive to EGFR-specific-siRNA. The combination of EGFR siRNA plus c-MET siRNA enhanced cell growth inhibition, apoptosis induction and inhibition of downstream signaling in EGFR TKI resistant H358, H1650 and H1975 cells, despite the absence of activity of the c-MET siRNA alone. EGFR TKIs or cetuximab plus su11274 were also consistently superior to either agent alone. The strongest biological effect was observed when afatinib, an irreversible pan-HER blocker was combined with su11274, which achieved a synergistic effect in the T790M mutant H1975 cells. In a conclusion, our findings offer preclinical proof of principle for combined inhibition as a promising treatment strategy for NSCLC, especially for patients in whom current EGFR-targeted treatments fail due to the presence of the T790M-EGFR-mutation or high c-MET expression.
Conflict of interest statement
Figures





References
-
- Lin WH, Song JS, Chang TY, Chang CY, Fu YN, et al. (2008) A cell-based high-throughput screen for epidermal growth factor receptor pathway inhibitors. Anal Biochem 377: 89–94. - PubMed
-
- Mendelsohn J, Baselga J (2003) Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 21: 2787–2799. - PubMed
-
- Zhou S, Ren S, Yan L, Zhang L, Tang L, et al. (2009) Clinical efficacy of erlotinib in patients previously treated for advanced non-small cell lung cancer. Respirology 14: 709–715. - PubMed
-
- Felip E, Rojo F, Reck M, Heller A, Klughammer B, et al. (2008) A phase II pharmacodynamic study of erlotinib in patients with advanced non-small cell lung cancer previously treated with platinum-based chemotherapy. Clin Cancer Res 14: 3867–3874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

