Generation and characterization of a novel mouse embryonic stem cell line with a dynamic reporter of Nanog expression
- PMID: 23527287
- PMCID: PMC3602340
- DOI: 10.1371/journal.pone.0059928
Generation and characterization of a novel mouse embryonic stem cell line with a dynamic reporter of Nanog expression
Erratum in
- PLoS One. 2014;9(6):e101028
Abstract
Background: The pluripotent state in embryonic stem (ES) cells is controlled by a core network of transcription factors that includes Nanog, Oct4 and Sox2. Nanog is required to reach pluripotency during somatic reprogramming and is the only core factor whose overexpression is able to oppose differentiation-promoting signals. Additionally, Nanog expression is known to fluctuate in ES cells, and different levels of Nanog seem to correlate with ES cells' ability to respond to differentiation promoting signals. Elucidating how dynamic Nanog levels are regulated in pluripotent cells and modulate their potential is therefore critical to develop a better understanding of the pluripotent state.
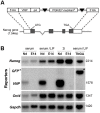
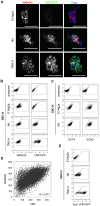
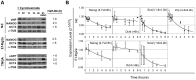
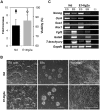
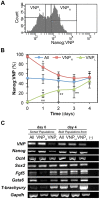
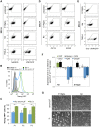
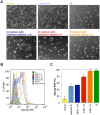
Methodology/principal findings: We describe the generation and validation of a mouse ES cell line with a novel Nanog reporter (Nd, from Nanog dynamics), containing a BAC transgene where the short-lived fluorescent protein VNP is placed under Nanog regulation. We show that Nanog and VNP have similar half-lives, and that Nd cells provide an accurate and measurable read-out for the dynamic levels of Nanog. Using this reporter, we could show that ES cells with low Nanog levels indeed have higher degree of priming to differentiation, when compared with high-Nanog cells. However, low-Nanog ES cells maintain high levels of Oct4 and Sox2 and can revert to a state of high-Nanog expression, indicating that they are still within the window of pluripotency. We further show that the observed changes in Nanog levels correlate with ES cell morphology and that Nanog dynamic expression is modulated by the cellular environment.
Conclusions/significance: The novel reporter ES cell line here described allows an accurate monitoring of Nanog's dynamic expression in the pluripotent state. This reporter will thus be a valuable tool to obtain quantitative measurements of global gene expression in pluripotent ES cells in different states, allowing a detailed molecular mapping of the pluripotency landscape.
Conflict of interest statement
Figures
References
-
- Potten CS, Loeffler M (1990) Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110: 1001–1020. - PubMed
-
- Morrison SJ, Shah NM, Anderson DJ (1997) Regulatory mechanisms in stem cell biology. Cell 88: 287–298. - PubMed
-
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292: 154–156. - PubMed
-
- Smith AG (2001) Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 17: 435–462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

