Alternating hemiplegia of childhood-related neural and behavioural phenotypes in Na+,K+-ATPase α3 missense mutant mice
- PMID: 23527305
- PMCID: PMC3603922
- DOI: 10.1371/journal.pone.0060141
Alternating hemiplegia of childhood-related neural and behavioural phenotypes in Na+,K+-ATPase α3 missense mutant mice
Abstract
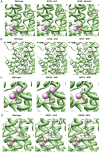
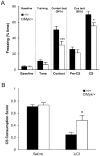
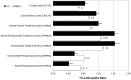
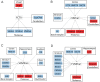
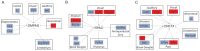
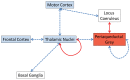
Missense mutations in ATP1A3 encoding Na(+),K(+)-ATPase α3 have been identified as the primary cause of alternating hemiplegia of childhood (AHC), a motor disorder with onset typically before the age of 6 months. Affected children tend to be of short stature and can also have epilepsy, ataxia and learning disability. The Na(+),K(+)-ATPase has a well-known role in maintaining electrochemical gradients across cell membranes, but our understanding of how the mutations cause AHC is limited. Myshkin mutant mice carry an amino acid change (I810N) that affects the same position in Na(+),K(+)-ATPase α3 as I810S found in AHC. Using molecular modelling, we show that the Myshkin and AHC mutations display similarly severe structural impacts on Na(+),K(+)-ATPase α3, including upon the K(+) pore and predicted K(+) binding sites. Behavioural analysis of Myshkin mice revealed phenotypic abnormalities similar to symptoms of AHC, including motor dysfunction and cognitive impairment. 2-DG imaging of Myshkin mice identified compromised thalamocortical functioning that includes a deficit in frontal cortex functioning (hypofrontality), directly mirroring that reported in AHC, along with reduced thalamocortical functional connectivity. Our results thus provide validation for missense mutations in Na(+),K(+)-ATPase α3 as a cause of AHC, and highlight Myshkin mice as a starting point for the exploration of disease mechanisms and novel treatments in AHC.
Conflict of interest statement
Figures

References
-
- Neville BG, Ninan M (2007) The treatment and management of alternating hemiplegia of childhood. Dev Med Child Neurol 49: 777–780. - PubMed
-
- Silver K, Andermann F (1993) Alternating hemiplegia of childhood: a study of 10 patients and results of flunarizine treatment. Neurology 43: 36–41. - PubMed
-
- Sweney MT, Silver K, Gerard-Blanluet M, Pedespan JM, Renault F, et al. (2009) Alternating hemiplegia of childhood: early characteristics and evolution of a neurodevelopmental syndrome. Pediatrics 123: e534–e541. - PubMed
-
- Panagiotakaki E, Gobbi G, Neville B, Ebinger F, Campistol J, et al. (2010) Evidence of a non-progressive course of alternating hemiplegia of childhood: study of a large cohort of children and adults. Brain 133: 3598–3610. - PubMed
-
- Sasaki M, Sakuma H, Fukushima A, Yamada K, Ohnishi T, et al. (2009) Abnormal cerebral glucose metabolism in alternating hemiplegia of childhood. Brain Dev 31: 20–26. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

