Self-assembly and conformational heterogeneity of the AXH domain of ataxin-1: an unusual example of a chameleon fold
- PMID: 23528090
- PMCID: PMC3602761
- DOI: 10.1016/j.bpj.2013.01.048
Self-assembly and conformational heterogeneity of the AXH domain of ataxin-1: an unusual example of a chameleon fold
Abstract
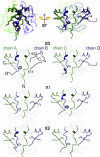
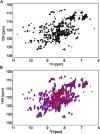
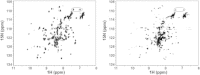
Ataxin-1 is a human protein responsible for spinocerebellar ataxia type 1, a hereditary disease associated with protein aggregation and misfolding. Essential for ataxin-1 aggregation is the anomalous expansion of a polyglutamine tract near the protein N-terminus, but the sequence-wise distant AXH domain modulates and contributes to the process. The AXH domain is also involved in the nonpathologic functions of the protein, including a variety of intermolecular interactions with other cellular partners. The domain forms a globular dimer in solution and displays a dimer of dimers arrangement in the crystal asymmetric unit. Here, we have characterized the domain further by studying its behavior in the crystal and in solution. We solved two new structures of the domain crystallized under different conditions that confirm an inherent plasticity of the AXH fold. In solution, the domain is present as a complex equilibrium mixture of monomeric, dimeric, and higher molecular weight species. This behavior, together with the tendency of the AXH fold to be trapped in local conformations, and the multiplicity of protomer interfaces, makes the AXH domain an unusual example of a chameleon protein whose properties bear potential relevance for the aggregation properties of ataxin-1 and thus for disease.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
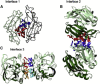


Similar articles
-
Protein-protein interactions as a strategy towards protein-specific drug design: the example of ataxin-1.PLoS One. 2013 Oct 14;8(10):e76456. doi: 10.1371/journal.pone.0076456. eCollection 2013. PLoS One. 2013. PMID: 24155902 Free PMC article.
-
The AXH domain adopts alternative folds the solution structure of HBP1 AXH.Structure. 2005 May;13(5):743-53. doi: 10.1016/j.str.2005.02.016. Structure. 2005. PMID: 15893665
-
A key lysine residue in the AXH domain of ataxin-1 is essential for its ubiquitylation.Biochim Biophys Acta. 2015 May;1854(5):356-64. doi: 10.1016/j.bbapap.2015.01.012. Epub 2015 Jan 29. Biochim Biophys Acta. 2015. PMID: 25641559
-
Kaleidoscopic protein-protein interactions in the life and death of ataxin-1: new strategies against protein aggregation.Trends Neurosci. 2014 Apr;37(4):211-8. doi: 10.1016/j.tins.2014.02.003. Epub 2014 Mar 11. Trends Neurosci. 2014. PMID: 24636457 Free PMC article. Review.
-
Molecular pathogenesis of spinocerebellar ataxia type 1 disease.Mol Cells. 2009 Jun 30;27(6):621-7. doi: 10.1007/s10059-009-0095-y. Epub 2009 Jun 22. Mol Cells. 2009. PMID: 19572115 Review.
Cited by
-
Modulation of the age at onset in spinocerebellar ataxia by CAG tracts in various genes.Brain. 2014 Sep;137(Pt 9):2444-55. doi: 10.1093/brain/awu174. Epub 2014 Jun 26. Brain. 2014. PMID: 24972706 Free PMC article.
-
Protein-protein interactions as a strategy towards protein-specific drug design: the example of ataxin-1.PLoS One. 2013 Oct 14;8(10):e76456. doi: 10.1371/journal.pone.0076456. eCollection 2013. PLoS One. 2013. PMID: 24155902 Free PMC article.
-
Toward the design and development of peptidomimetic inhibitors of the Ataxin-1 aggregation pathway.Biophys J. 2022 Dec 6;121(23):4679-4688. doi: 10.1016/j.bpj.2022.10.021. Epub 2022 Oct 19. Biophys J. 2022. PMID: 36262042 Free PMC article.
-
Chaperones in Polyglutamine Aggregation: Beyond the Q-Stretch.Front Neurosci. 2017 Mar 23;11:145. doi: 10.3389/fnins.2017.00145. eCollection 2017. Front Neurosci. 2017. PMID: 28386214 Free PMC article. Review.
-
Chemical shift assignment of the ataxin-1 AXH domain in complex with a CIC ligand peptide.Biomol NMR Assign. 2014 Oct;8(2):325-7. doi: 10.1007/s12104-013-9509-z. Epub 2013 Jul 14. Biomol NMR Assign. 2014. PMID: 23853075 Free PMC article.
References
-
- Orr H.T., Chung M.Y., Zoghbi H.Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 1993;4:221–226. - PubMed
-
- Matilla-Dueñas A., Goold R., Giunti P. Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum. 2008;7:106–114. - PubMed
-
- Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. - PubMed
-
- Soto C., Estrada L.D. Protein misfolding and neurodegeneration. Arch. Neurol. 2008;65:184–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

