Minimal models for cell-cycle control based on competitive inhibition and multisite phosphorylations of Cdk substrates
- PMID: 23528096
- PMCID: PMC3602763
- DOI: 10.1016/j.bpj.2013.02.012
Minimal models for cell-cycle control based on competitive inhibition and multisite phosphorylations of Cdk substrates
Abstract
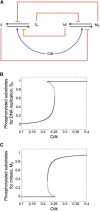
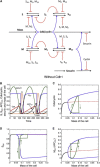
The eukaryotic cell cycle is characterized by alternating oscillations in the activities of cyclin-dependent kinase (Cdk) and the anaphase-promoting complex (APC). Successful completion of the cell cycle is dependent on the precise, temporally ordered appearance of these activities. A modest level of Cdk activity is sufficient to initiate DNA replication, but mitosis and APC activation require an elevated Cdk activity. In present-day eukaryotes, this temporal order is provided by a complex network of regulatory proteins that control both Cdk and APC activities via sharp thresholds, bistability, and time delays. Using simple computational models, we show here that these dynamical features of cell-cycle organization could emerge in a control system driven by a single Cdk/cyclin complex and APC wired in a negative-feedback loop. We show that ordered phosphorylation of cellular proteins could be explained by multisite phosphorylation/dephosphorylation and competition of substrates for interconverting kinase (Cdk) and phosphatase. In addition, the competition of APC substrates for ubiquitylation can create and maintain sustained oscillations in cyclin levels. We propose a sequence of models that gets closer and closer to a realistic model of cell-cycle control in yeast. Since these models lack the elaborate control mechanisms characteristic of modern eukaryotes, they suggest that bistability and time delay may have characterized eukaryotic cell divisions before the current cell-cycle control network evolved in all its complexity.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Mitotic exit in two dimensions.J Theor Biol. 2007 Oct 7;248(3):560-73. doi: 10.1016/j.jtbi.2007.06.014. Epub 2007 Jun 17. J Theor Biol. 2007. PMID: 17659305
-
Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14.Curr Biol. 1999 Mar 11;9(5):227-36. doi: 10.1016/s0960-9822(99)80111-0. Curr Biol. 1999. PMID: 10074450
-
Securin and B-cyclin/CDK are the only essential targets of the APC.Nat Cell Biol. 2003 Dec;5(12):1090-4. doi: 10.1038/ncb1066. Epub 2003 Nov 23. Nat Cell Biol. 2003. PMID: 14634663
-
Finishing mitosis, one step at a time.Nat Rev Mol Cell Biol. 2007 Nov;8(11):894-903. doi: 10.1038/nrm2276. Nat Rev Mol Cell Biol. 2007. PMID: 17912263 Review.
-
Cdk-counteracting phosphatases unlock mitotic exit.Curr Opin Cell Biol. 2008 Dec;20(6):661-8. doi: 10.1016/j.ceb.2008.09.003. Epub 2008 Oct 22. Curr Opin Cell Biol. 2008. PMID: 18845253 Free PMC article. Review.
Cited by
-
Network mechanisms and dysfunction within an integrated computational model of progression through mitosis in the human cell cycle.PLoS Comput Biol. 2020 Apr 6;16(4):e1007733. doi: 10.1371/journal.pcbi.1007733. eCollection 2020 Apr. PLoS Comput Biol. 2020. PMID: 32251461 Free PMC article.
-
Cyclin/Forkhead-mediated coordination of cyclin waves: an autonomous oscillator rationalizing the quantitative model of Cdk control for budding yeast.NPJ Syst Biol Appl. 2021 Dec 13;7(1):48. doi: 10.1038/s41540-021-00201-w. NPJ Syst Biol Appl. 2021. PMID: 34903735 Free PMC article. Review.
-
Enhancement of tunability of MAPK cascade due to coexistence of processive and distributive phosphorylation mechanisms.Biophys J. 2014 Mar 4;106(5):1215-26. doi: 10.1016/j.bpj.2014.01.036. Biophys J. 2014. PMID: 24606945 Free PMC article.
-
The formation of tight tumor clusters affects the efficacy of cell cycle inhibitors: a hybrid model study.J Theor Biol. 2014 Jul 7;352:31-50. doi: 10.1016/j.jtbi.2014.02.027. Epub 2014 Mar 5. J Theor Biol. 2014. PMID: 24607745 Free PMC article.
-
Computer-Aided Whole-Cell Design: Taking a Holistic Approach by Integrating Synthetic With Systems Biology.Front Bioeng Biotechnol. 2020 Aug 7;8:942. doi: 10.3389/fbioe.2020.00942. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32850764 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

