Radiosynthesis and biological evaluation of alpha-[F-18]fluoromethyl phenylalanine for brain tumor imaging
- PMID: 23528560
- PMCID: PMC3644424
- DOI: 10.1016/j.nucmedbio.2012.12.013
Radiosynthesis and biological evaluation of alpha-[F-18]fluoromethyl phenylalanine for brain tumor imaging
Abstract
Objectives: Radiolabeled amino acids have proven utility for imaging brain tumors in humans, particularly those that target system L amino acid transport. We have prepared the novel phenylalanine analogue, (FMePhe, 9), as part of an effort to develop new system L tracers that can be prepared in high radiochemical yield through nucleophilic [(18)F]fluorination. The tumor imaging properties of both enantiomers of this new tracer were evaluated through cell uptake, biodistribution and microPET studies in the mouse DBT model of high grade glioma.
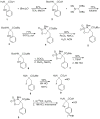
Methods: The non-radioactive form of 9 and the cyclic sulfamidate labeling precursor were prepared from commercially available racemic α-benzylserine. Racemic [(18)F]9 was prepared from the labeling precursor in two steps using standard[(18)F]fluoride nucleophilic reaction conditions followed by acidic deprotection. The individual enantiomers [(18)F]9a and [(18)F]9b were isolated using preparative chiral HPLC. In vitro uptake inhibition assays were performed with each enantiomer using DBT cells. Biodistribution and microPET/CT studies were performed with each enantiomer in male BALB/c mice at approximately 2 weeks after implantation of DBT tumor cells.
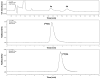
Results: Radiolabeling of the cyclic sulfamidate precursor 5 provides racemic [(18)F]9 in high radiochemical yield (60%-70%, n=4) and high radiochemical purity (>96%, n=4). In vitro uptake assays demonstrate that both [(18)F]9a and [(18)F]9b undergo tumor cell uptake through system L transport. The biodistribution studies using the single enantiomers [(18)F]9a and [(18)F]9b demonstrated good tumor uptake with lower uptake in most normal tissues, and [(18)F]9a had higher tumor uptake than [(18)F]9b. MicroPET imaging demonstrated good tumor visualization within 10 min of injection, rapid uptake of radioactivity, and tumor to brain ratios of approximately 6:1 at 60 min postinjection.
Conclusions: The novel PET tracer, [(18)F]FMePhe, is readily synthesized in good yield from a cyclic sulfamidate precursor. Biodistribution and microPET studies in the DBT model demonstrate good tumor to tissue ratios and tumor visualization, with enantiomer [(18)F]9a having higher tumor uptake. However, the brain availability of both enantiomers was lower than expected for system L substrates, suggesting the [(18)F]fluorine group in the β-position affects uptake of these compounds by system L transporters.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






References
-
- Langen KJ, Pauleit D, Coenen HH. 3-[123I]Iodo-alpha-methyl-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. 2002;29:625–31. - PubMed
-
- Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D, et al. O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. 2006;33:287–94. - PubMed
-
- Sutinen E, Jyrkkio S, Alanen K, Nagren K, Minn H. Uptake of [N-methyl-11C]alpha-methylaminoisobutyric acid in untreated head and neck cancer studied by PET. Eur J Nucl Med Mol Imaging. 2003;30:72–7. - PubMed
-
- McConathy J, Yu W, Jarkas N, Seo W, Schuster DM, Goodman MM. Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med Res Rev. 2011;26:20250. - PubMed
-
- Qu W, Zha Z, Ploessl K, Lieberman BP, Zhu L, Wise DR, et al. Synthesis of optically pure 4-fluoro-glutamines as potential metabolic imaging agents for tumors. J Am Chem Soc. 2011;133:1122–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

