Structure of the tubulin/FtsZ-like protein TubZ from Pseudomonas bacteriophage ΦKZ
- PMID: 23528827
- PMCID: PMC3678025
- DOI: 10.1016/j.jmb.2013.03.019
Structure of the tubulin/FtsZ-like protein TubZ from Pseudomonas bacteriophage ΦKZ
Abstract
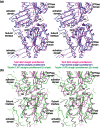
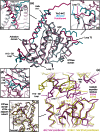
Pseudomonas ΦKZ-like bacteriophages encode a group of related tubulin/FtsZ-like proteins believed to be essential for the correct centring of replicated bacteriophage virions within the bacterial host. In this study, we present crystal structures of the tubulin/FtsZ-like protein TubZ from Pseudomonas bacteriophage ΦKZ in both the monomeric and protofilament states, revealing that ΦKZ TubZ undergoes structural changes required to polymerise, forming a canonical tubulin/FtsZ-like protofilament. Combining our structures with previous work, we propose a polymerisation-depolymerisation cycle for the Pseudomonas bacteriophage subgroup of tubulin/FtsZ-like proteins. Electron cryo-microscopy of ΦKZ TubZ filaments polymerised in vitro implies a long-pitch helical arrangement for the constituent protofilaments. Intriguingly, this feature is shared by the other known subgroup of bacteriophage tubulin/FtsZ-like proteins from Clostridium species, which are thought to be involved in partitioning the genomes of bacteriophages adopting a pseudo-lysogenic life cycle.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Bergh O., Børsheim K.Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. - PubMed
-
- Löwe J., Amos L.A. Evolution of cytomotive filaments: the cytoskeleton from prokaryotes to eukaryotes. Int. J. Biochem. Cell Biol. 2009;41:323–329. - PubMed
-
- Aylett C.H.S., Löwe J., Amos L.A. New insights into the mechanisms of cytomotive actin and tubulin filaments. Int. Rev. Cell Mol. Biol. 2011;292:1–71. - PubMed
-
- Austin S., Abeles A. Partition of unit-copy miniplasmids to daughter cells. I. P1 and F miniplasmids contain discrete, interchangeable sequences sufficient to promote equipartition. J. Mol. Biol. 1983;169:353–372. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

