Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy
- PMID: 23529375
- PMCID: PMC3631110
- DOI: 10.1007/s11910-013-0347-2
Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy
Abstract
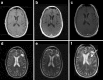
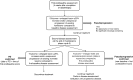
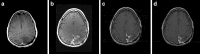
Since 1990, the primary criteria used for assessing response to therapy in high-grade gliomas were those developed by Macdonald and colleagues, which incorporated 2-dimensional area measurements of contrast-enhancing tumor regions, corticosteroid dosing, and clinical assessment to arrive at a designation of response, stable disease, or progression. Recent advances in imaging technology and targeted therapeutics, however, have exposed limitations of the Macdonald criteria and have highlighted the need for reevaluation of response assessment criteria. In 2010, the Response Assessment in Neuro-Oncology (RANO) Working Group published updated criteria to address this need and to standardize response assessment for high-grade gliomas. In 2009, prior to the publication of the RANO criteria, the randomized, placebo-controlled, multicenter, phase 3 AVAglio trial was designed and initiated to investigate the effectiveness of radiotherapy and temozolomide with or without bevacizumab in newly diagnosed glioblastoma. The AVAglio protocol enacted specific measures to adapt the Macdonald criteria to the frontline treatment setting and to antiangiogenic agent evaluation, including the incorporation of a T2/fluid-attenuated inversion recovery component, qualitative assessment of irregularly shaped contrast-enhancing lesions, and a decision tree for confirming or ruling out pseudoprogression. Moreover, the protocol outlines practical means by which these adapted response criteria can be implemented in the clinic. This article describes the evolution of radiographic response criteria for high-grade gliomas and highlights the similarities and differences between those implemented in the AVAglio study and those subsequently published by RANO.
Trial registration: ClinicalTrials.gov NCT00943826.
Conflict of interest statement
Olivier L. Chinot has been a paid consultant for F. Hoffmann-La Roche and received honoraria from Astra-Zeneca and MSD.
David R. Macdonald has received honoraria and travel support from Merck Canada, MSD, and F. Hoffmann-La Roche Canada, and travel support (to attend investigators' meetings) from EMD Serono and Celldex Therapeutics.
Lauren E. Abrey is an employee of F. Hoffmann-La Roche.
Gudrun Zahlmann is an employee of F. Hoffmann-La Roche.
Yannick Kerloëguen is an employee of F. Hoffmann-La Roche.
Timothy F. Cloughesy has received consulting honoraria from F. Hoffmann-La Roche, Genentech, Celgene, Merck, Merck Serono, and participated in a speakers’ bureau for Merck.
Figures

References
-
- CBTRUS. (2011) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. http://www.cbtrus.org/2011-NPCR-SEER/WEB-0407-Report-3-3-2011.pdf. Accessed 24 January 2013.
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide vs radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

