Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx
- PMID: 23529621
- PMCID: PMC3676016
- DOI: 10.1128/IAI.00084-13
Clearance of Staphylococcus aureus nasal carriage is T cell dependent and mediated through interleukin-17A expression and neutrophil influx
Abstract
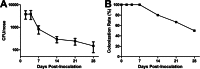
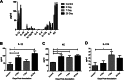
The anterior nares of humans are the major reservoir for Staphylococcus aureus colonization. Approximately 20% of the healthy human population is persistently and 80% is intermittently colonized with S. aureus in the nasal cavity. Previous studies have shown a strong causal connection between S. aureus nasal carriage and increased risk of nosocomial infection, as well as increased carriage due to immune dysfunction. However, the immune responses that permit persistence or mediate clearance of S. aureus on the nasal mucosa are fundamentally undefined. In this study, we developed a carriage model in C57BL/6J mice and showed that clearance begins 14 days postinoculation. In contrast, SCID mice that have a deficient adaptive immune response are unable to eliminate S. aureus even after 28 days postinoculation. Furthermore, decolonization was found to be T cell mediated but B cell independent by evaluating carriage clearance in T-cell receptor β/δ (TCR-β/δ) knockout (KO) and IgH-μ KO mice, respectively. Upregulation of the cytokines interleukin 1β (IL-1β), KC (also termed CXC ligand 1 [CXCL1]), and IL-17A occurred following inoculation with intranasal S. aureus. IL-17A production was crucial for clearance, since IL-17A-deficient mice were unable to effectively eliminate S. aureus carriage. Subsequently, cell differential counts were evaluated from nasal lavage fluid obtained from wild-type and IL-17A-deficient colonized mice. These counts displayed IL-17A-dependent neutrophil migration. Antibody-mediated depletion of neutrophils in colonized mice caused reduced clearance compared to that in isotype-treated controls. Our data suggest that the Th17-associated immune response is required for nasal decolonization. This response is T cell dependent and mediated via IL-17A production and neutrophil influx. Th17-associated immune responses may be targeted for strategies to mitigate distal infections originating from persistent S. aureus carriage in humans.
Figures



References
-
- von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11–16 - PubMed
-
- NNIS System 1999. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1990-May 1999, issued June 1999. Am. J. Infect. Control 27:520–532 - PubMed
-
- Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705 - PubMed
-
- Hu L, Umeda A, Kondo S, Amako K. 1995. Typing of Staphylococcus aureus colonising human nasal carriers by pulsed-field gel electrophoresis. J. Med. Microbiol. 42:127–132 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

