Pharmacometric characterization of dabigatran hemodialysis
- PMID: 23529813
- PMCID: PMC3663980
- DOI: 10.1007/s40262-013-0049-6
Pharmacometric characterization of dabigatran hemodialysis
Abstract
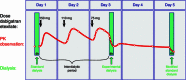
Background: Hemodialysis has been shown to be a useful method of decreasing dabigatran plasma levels in situations that require rapid elimination of this thrombin inhibitor. However, there is currently no clinical recommendation for the accelerated/optimized elimination of dabigatran via hemodialysis (e.g., flow rates, filter type, duration of dialysis).
Objectives: The primary objective of the present work was to characterize, via pharmacometric methods, the effects of different blood flow rates in hemodialysis on the pharmacokinetics of dabigatran, using data from a dedicated phase I dialysis study of end-stage renal disease (ESRD) patients. In addition, the effects of various clinically relevant hemodialysis settings were evaluated by simulation to assess their potential use in non-ESRD situations.
Methods: Seven patients with ESRD were investigated in an open-label, fixed-sequence, two-period comparison trial. A population pharmacokinetic model was developed to fit the data and then used for various simulations. Data analyses were performed using NONMEM(®), Berkeley Madonna, or SAS.
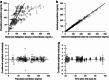
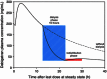
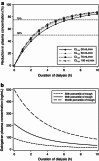
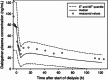
Results: The pharmacokinetics of dabigatran were best described by a two-compartment model with first-order absorption and a lag time. In addition to total body clearance in ESRD subjects, a first-order dialysis clearance was implemented which was greater than zero during hemodialysis and zero during the interdialytic periods. The relationship between the dialysis clearance and the blood flow rate was best described by the Michaels function. Simulations showed that varying clinically relevant dialysis settings such as filter properties or flow rates had only minor effects. Dialysis duration had the strongest impact on dabigatran plasma concentration. The observed geometric mean redistribution effect after hemodialysis was low (<16 %). The final model was successfully evaluated through the prediction of plasma concentrations from a case report undergoing dialysis.
Conclusions: This analysis allowed the influences of various hemodialysis parameters on the dabigatran plasma concentration to be predicted in detail for the first time. Dialysis duration was identified as having the strongest impact on the reduction in dabigatran plasma concentration. The model developed here can potentially serve as a tool to provide guidance when considering the use of hemodialysis in patients who have received dabigatran.
Figures


References
-
- Van de Werf F, Brueckmann M, Connolly SJ, et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: The Randomized, phase II study to Evaluate the sAfety and pharmacokinetics of oraL dabIGatran etexilate in patients after heart valve replacemeNt (RE-ALIGN) Am Heart J. 2012;163:931–937. doi: 10.1016/j.ahj.2012.03.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

