Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes
- PMID: 23530060
- PMCID: PMC3648076
- DOI: 10.1128/MCB.01004-12
Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes
Abstract
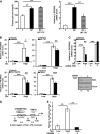
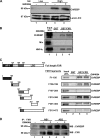
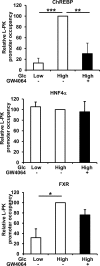
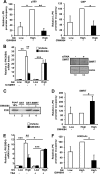
The glucose-activated transcription factor carbohydrate response element binding protein (ChREBP) induces the expression of hepatic glycolytic and lipogenic genes. The farnesoid X receptor (FXR) is a nuclear bile acid receptor controlling bile acid, lipid, and glucose homeostasis. FXR negatively regulates hepatic glycolysis and lipogenesis in mouse liver. The aim of this study was to determine whether FXR regulates the transcriptional activity of ChREBP in human hepatocytes and to unravel the underlying molecular mechanisms. Agonist-activated FXR inhibits glucose-induced transcription of several glycolytic genes, including the liver-type pyruvate kinase gene (L-PK), in the immortalized human hepatocyte (IHH) and HepaRG cell lines. This inhibition requires the L4L3 region of the L-PK promoter, known to bind the transcription factors ChREBP and hepatocyte nuclear factor 4α (HNF4α). FXR interacts directly with ChREBP and HNF4α proteins. Analysis of the protein complex bound to the L4L3 region reveals the presence of ChREBP, HNF4α, FXR, and the transcriptional coactivators p300 and CBP at high glucose concentrations. FXR activation does not affect either FXR or HNF4α binding to the L4L3 region but does result in the concomitant release of ChREBP, p300, and CBP and in the recruitment of the transcriptional corepressor SMRT. Thus, FXR transrepresses the expression of genes involved in glycolysis in human hepatocytes.
Figures



References
-
- Bouché C, Serdy S, Kahn CR, Goldfine AB. 2004. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr. Rev. 25:807–830 - PubMed
-
- Nordlie RC, Foster JD, Lange AJ. 1999. Regulation of glucose production by the liver. Annu. Rev. Nutr. 19:379–406 - PubMed
-
- Girard J, Ferré P, Foufelle F. 1997. Mechanisms by which carbohydrates regulate expression of genes for glycolytic and lipogenic enzymes. Annu. Rev. Nutr. 17:325–352 - PubMed
-
- de Luis O, Valero MC, Jurado LA. 2000. WBSCR14, a putative transcription factor gene deleted in Williams-Beuren syndrome: complete characterisation of the human gene and the mouse ortholog. Eur. J. Hum. Genet. 8:215–222 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
