An in vivo drug screening model using glucose-6-phosphate dehydrogenase deficient mice to predict the hemolytic toxicity of 8-aminoquinolines
- PMID: 23530079
- PMCID: PMC3752814
- DOI: 10.4269/ajtmh.12-0682
An in vivo drug screening model using glucose-6-phosphate dehydrogenase deficient mice to predict the hemolytic toxicity of 8-aminoquinolines
Abstract
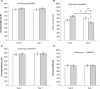
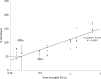
Anti-malarial 8-aminoquinolines drugs cause acute hemolytic anemia in individuals with glucose-6-phosphate dehydrogenase deficiency (G6PDD). Efforts to develop non-hemolytic 8-aminoquinolines have been severely limited caused by the lack of a predictive in vivo animal model of hemolytic potential that would allow screening of candidate compounds. This report describes a G6PDD mouse model with a phenotype closely resembling the G6PDD phenotype found in the African A-type G6PDD human. These G6PDD mice, given different doses of primaquine, which used as a reference hemolytic drug, display a full array of hemolytic anemia parameters, consistently and reproducibly. The hemolytic and therapeutic indexes were generated for evaluation of hemotoxicity of drugs. This model demonstrated a complete hemolytic toxicity response to another known hemolytic antimalarial drug, pamaquine, but no response to non-hemolytic drugs, chloroquine and mefloquine. These results suggest that this model is suitable for evaluation of selected 8-AQ type candidate antimalarial drugs for their hemolytic potential.
Figures


References
-
- Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. - PubMed
-
- Beutler E. G6PD: population genetics and clinical manifestations. Blood Rev. 1996;10:45–52. - PubMed
-
- Beutler E. The hemolytic effect of primaquine and related compounds: a review. Blood. 1959;14:103–139. - PubMed
-
- Baird JK, Fryauff DJ, Basri H, Bangs MJ, Subianto B, Wiady I, Purnomo, Leksana B, Masbar S, Richie TL, Jones TR, Tjitra E, Wignall ES, Hoffman SL. Primaquine for prophylaxis against malaria among nonimmune transmigrants in Irian Jaya, Indonesia. Am J Trop Med Hyg. 1995;52:479–484. - PubMed
-
- Baird JK, Fryauff DJ, Hoffman SL. Primaquine for prevention of malaria in travelers. Clin Infect Dis. 2003;37:1659–1667. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

