Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation
- PMID: 23530145
- PMCID: PMC3633638
- DOI: 10.4049/jimmunol.1201742
Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation
Abstract
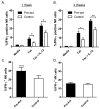
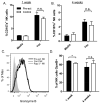
Several recent studies have demonstrated that innate immune NK cells exhibit memory-like properties with enhanced nonspecific and specific recall responses. Cytokine activation alone of murine NK cells induces the differentiation of memory-like cells that are more likely to produce IFN-γ, a key NK cell cytokine important for activation of the immune response. Using an adoptive cotransfer system, we first show that cytokine-induced memory-like responses are NK intrinsic. However, engraftment of donor NK cells in NK-competent hosts is poor because of homeostatic control mechanisms. Therefore, we used alymphoid Rag- and common γ-chain-deficient mice as recipients and observed homeostatic expansion of cotransferred cytokine-activated and control donor NK cells. Despite proliferation of all cells, NK cells derived from those cells originally activated by cytokines retained an intrinsic enhanced capacity to produce IFN-γ when restimulated in vitro with cytokines or target cells. These NK cell memory-like responses persisted for at least 4 wk in alymphoid hosts and 12 wk in NK-competent hosts. These findings indicate that memory-like NK cells can readily self-renew and maintain enhanced function in a lymphopenic host for at least a month.
Figures



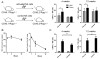
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

