In vitro and in vivo delivery of genes and proteins using the bacteriophage T4 DNA packaging machine
- PMID: 23530211
- PMCID: PMC3625312
- DOI: 10.1073/pnas.1300867110
In vitro and in vivo delivery of genes and proteins using the bacteriophage T4 DNA packaging machine
Abstract
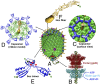
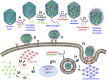
The bacteriophage T4 DNA packaging machine consists of a molecular motor assembled at the portal vertex of an icosahedral head. The ATP-powered motor packages the 56-µm-long, 170-kb viral genome into 120 nm × 86 nm head to near crystalline density. We engineered this machine to deliver genes and proteins into mammalian cells. DNA molecules were translocated into emptied phage head and its outer surface was decorated with proteins fused to outer capsid proteins, highly antigenic outer capsid protein (Hoc) and small outer capsid protein (Soc). T4 nanoparticles carrying reporter genes, vaccine candidates, functional enzymes, and targeting ligands were efficiently delivered into cells or targeted to antigen-presenting dendritic cells, and the delivered genes were abundantly expressed in vitro and in vivo. Mice delivered with a single dose of F1-V plague vaccine containing both gene and protein in the T4 head elicited robust antibody and cellular immune responses. This "progene delivery" approach might lead to new types of vaccines and genetic therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Structure, assembly, and DNA packaging of the bacteriophage T4 head.Adv Virus Res. 2012;82:119-53. doi: 10.1016/B978-0-12-394621-8.00018-2. Adv Virus Res. 2012. PMID: 22420853 Free PMC article. Review.
-
Portal fusion protein constraints on function in DNA packaging of bacteriophage T4.Mol Microbiol. 2006 Jul;61(1):16-32. doi: 10.1111/j.1365-2958.2006.05203.x. Mol Microbiol. 2006. PMID: 16824092
-
A Bacteriophage T4 Nanoparticle-Based Dual Vaccine against Anthrax and Plague.mBio. 2018 Oct 16;9(5):e01926-18. doi: 10.1128/mBio.01926-18. mBio. 2018. PMID: 30327445 Free PMC article.
-
Mutated and bacteriophage T4 nanoparticle arrayed F1-V immunogens from Yersinia pestis as next generation plague vaccines.PLoS Pathog. 2013;9(7):e1003495. doi: 10.1371/journal.ppat.1003495. Epub 2013 Jul 11. PLoS Pathog. 2013. PMID: 23853602 Free PMC article.
-
Structure and assembly of bacteriophage T4 head.Virol J. 2010 Dec 3;7:356. doi: 10.1186/1743-422X-7-356. Virol J. 2010. PMID: 21129201 Free PMC article. Review.
Cited by
-
Antigen self-anchoring onto bacteriophage T5 capsid-like particles for vaccine design.NPJ Vaccines. 2024 Jan 4;9(1):6. doi: 10.1038/s41541-023-00798-5. NPJ Vaccines. 2024. PMID: 38177231 Free PMC article.
-
Viral nanoparticle-encapsidated enzyme and restructured DNA for cell delivery and gene expression.Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13319-24. doi: 10.1073/pnas.1321940111. Epub 2014 Aug 26. Proc Natl Acad Sci U S A. 2014. PMID: 25161284 Free PMC article.
-
Geometric architecture of viruses.World J Virol. 2020 Aug 25;9(2):5-18. doi: 10.5501/wjv.v9.i2.5. World J Virol. 2020. PMID: 32923381 Free PMC article. Review.
-
Plague Vaccines: Status and Future.Adv Exp Med Biol. 2016;918:313-360. doi: 10.1007/978-94-024-0890-4_12. Adv Exp Med Biol. 2016. PMID: 27722869 Free PMC article. Review.
-
Genetic Engineering of Bacteriophages Against Infectious Diseases.Front Microbiol. 2019 May 3;10:954. doi: 10.3389/fmicb.2019.00954. eCollection 2019. Front Microbiol. 2019. PMID: 31130936 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

