Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide
- PMID: 23530213
- PMCID: PMC3625313
- DOI: 10.1073/pnas.1222607110
Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide
Abstract
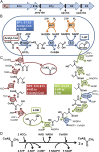
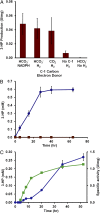
Microorganisms can be engineered to produce useful products, including chemicals and fuels from sugars derived from renewable feedstocks, such as plant biomass. An alternative method is to use low potential reducing power from nonbiomass sources, such as hydrogen gas or electricity, to reduce carbon dioxide directly into products. This approach circumvents the overall low efficiency of photosynthesis and the production of sugar intermediates. Although significant advances have been made in manipulating microorganisms to produce useful products from organic substrates, engineering them to use carbon dioxide and hydrogen gas has not been reported. Herein, we describe a unique temperature-dependent approach that confers on a microorganism (the archaeon Pyrococcus furiosus, which grows optimally on carbohydrates at 100°C) the capacity to use carbon dioxide, a reaction that it does not accomplish naturally. This was achieved by the heterologous expression of five genes of the carbon fixation cycle of the archaeon Metallosphaera sedula, which grows autotrophically at 73°C. The engineered P. furiosus strain is able to use hydrogen gas and incorporate carbon dioxide into 3-hydroxypropionic acid, one of the top 12 industrial chemical building blocks. The reaction can be accomplished by cell-free extracts and by whole cells of the recombinant P. furiosus strain. Moreover, it is carried out some 30°C below the optimal growth temperature of the organism in conditions that support only minimal growth but maintain sufficient metabolic activity to sustain the production of 3-hydroxypropionate. The approach described here can be expanded to produce important organic chemicals, all through biological activation of carbon dioxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Somerville C, Youngs H, Taylor C, Davis SC, Long SP. Feedstocks for lignocellulosic biofuels. Science. 2010;329(5993):790–792. - PubMed
-
- Olson DG, McBride JE, Shaw AJ, Lynd LR. Recent progress in consolidated bioprocessing. Curr Opin Biotechnol. 2012;23(3):396–405. - PubMed
-
- Steen EJ, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature. 2010;463(7280):559–562. - PubMed
-
- Peralta-Yahya PP, Keasling JD. Advanced biofuel production in microbes. Biotechnol J. 2010;5(2):147–162. - PubMed
-
- Wackett LP. Engineering microbes to produce biofuels. Curr Opin Biotechnol. 2011;22(3):388–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

