Hypoxia induces a phase transition within a kinase signaling network in cancer cells
- PMID: 23530221
- PMCID: PMC3625329
- DOI: 10.1073/pnas.1303060110
Hypoxia induces a phase transition within a kinase signaling network in cancer cells
Abstract
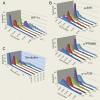
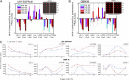
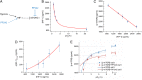
Hypoxia is a near-universal feature of cancer, promoting glycolysis, cellular proliferation, and angiogenesis. The molecular mechanisms of hypoxic signaling have been intensively studied, but the impact of changes in oxygen partial pressure (pO2) on the state of signaling networks is less clear. In a glioblastoma multiforme (GBM) cancer cell model, we examined the response of signaling networks to targeted pathway inhibition between 21% and 1% pO2. We used a microchip technology that facilitates quantification of a panel of functional proteins from statistical numbers of single cells. We find that near 1.5% pO2, the signaling network associated with mammalian target of rapamycin (mTOR) complex 1 (mTORC1)--a critical component of hypoxic signaling and a compelling cancer drug target--is deregulated in a manner such that it will be unresponsive to mTOR kinase inhibitors near 1.5% pO2, but will respond at higher or lower pO2 values. These predictions were validated through experiments on bulk GBM cell line cultures and on neurosphere cultures of a human-origin GBM xenograft tumor. We attempt to understand this behavior through the use of a quantitative version of Le Chatelier's principle, as well as through a steady-state kinetic model of protein interactions, both of which indicate that hypoxia can influence mTORC1 signaling as a switch. The Le Chatelier approach also indicates that this switch may be thought of as a type of phase transition. Our analysis indicates that certain biologically complex cell behaviors may be understood using fundamental, thermodynamics-motivated principles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447. - PubMed
-
- Collingridge DR, Piepmeier JM, Rockwell S, Knisely JP. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother Oncol. 1999;53(2):127–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

