Strong subunit coordination drives a powerful viral DNA packaging motor
- PMID: 23530228
- PMCID: PMC3625343
- DOI: 10.1073/pnas.1222820110
Strong subunit coordination drives a powerful viral DNA packaging motor
Abstract
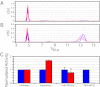
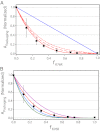
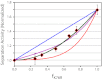
Terminase enzymes are viral motors that package DNA into a preformed capsid and are of interest both therapeutically and as potential nano-machines. The enzymes excise a single genome from a concatemeric precursor (genome maturation) and then package the duplex to near-crystalline density (genome packaging). The functional motors are oligomers of protomeric subunits and are the most powerful motors currently known. Here, we present mechanistic studies on the terminase motor from bacteriophage λ. We identify a mutant (K76R) that is specifically deficient in packaging activity. Biochemical analysis of this enzyme provides insight into the linkage between ATP hydrolysis and motor translocation. We further use this mutant to assemble chimeric motors with WT enzyme and characterize the catalytic activity of the complexes. The data demonstrate that strong coordination between the motor protomers is required for DNA packaging and that incorporation of even a single mutant protomer poisons motor activity. Significant coordination is similarly observed in the genome maturation reaction; however, although the motor is composed of a symmetric tetramer of protomers, the maturation complex is better described as a "dimer-of-dimers" with half-site reactivity. We describe a model for how the motor alternates between a stable genome maturation complex and a dynamic genome packaging complex. The fundamental features of coordinated ATP hydrolysis, DNA movement, and tight association between the motor and the duplex during translocation are recapitulated in all of the viral motors. This work is thus of relevance to all terminase enzymes, both prokaryotic and eukaryotic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
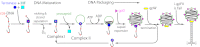
References
-
- Calendar R, Abedon ST. The Bacteriophages. New York: Oxford Univ Press; 2006.
-
- Knipe DM, Howley PM. Fields Virology. 5th Ed. New York: Lippincott-Williams, and Wilkins; 2007.
-
- Catalano CE. Viral genome packaging machines: An overview. In: Catalano CE, editor. Viral Genome Packaging Machines: Genetics, Structure, and Mechanism. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 1–4.
-
- Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. New York, NY: Lippincott, Williams, and Wilkins; 2007. pp. 2501–2602.
-
- Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu Rev Genet. 2008;42:647–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

