Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation
- PMID: 23530233
- PMCID: PMC3625351
- DOI: 10.1073/pnas.1213294110
Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation
Abstract
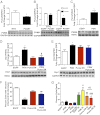
Mitochondrial morphological dynamics affect the outcome of ischemic heart damage and pathogenesis. Recently, mitochondrial fission protein dynamin-related protein 1 (Drp1) has been identified as a mediator of mitochondrial morphological changes and cell death during cardiac ischemic injury. In this study, we report a unique relationship between Pim-1 activity and Drp1 regulation of mitochondrial morphology in cardiomyocytes challenged by ischemic stress. Transgenic hearts overexpressing cardiac Pim-1 display reduction of total Drp1 protein levels, increased phosphorylation of Drp1-(S637), and inhibition of Drp1 localization to the mitochondria. Consistent with these findings, adenoviral-induced Pim-1 neonatal rat cardiomyocytes (NRCMs) retain a reticular mitochondrial phenotype after simulated ischemia (sI) and decreased Drp1 mitochondrial sequestration. Interestingly, adenovirus Pim-dominant negative NRCMs show increased expression of Bcl-2 homology 3 (BH3)-only protein p53 up-regulated modulator of apoptosis (PUMA), which has been previously shown to induce Drp1 accumulation at mitochondria and increase sensitivity to apoptotic stimuli. Overexpression of the p53 up-regulated modulator of apoptosis-dominant negative adenovirus attenuates localization of Drp1 to mitochondria in adenovirus Pim-dominant negative NRCMs promotes reticular mitochondrial morphology and inhibits cell death during sI. Therefore, Pim-1 activity prevents Drp1 compartmentalization to the mitochondria and preserves reticular mitochondrial morphology in response to sI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Machado NG, Alves MG, Carvalho RA, Oliveira PJ. Mitochondrial involvement in cardiac apoptosis during ischemia and reperfusion: Can we close the box? Cardiovasc Toxicol. 2009;9(4):211–227. - PubMed
-
- Ong SB, et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–2022. - PubMed
-
- Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17(11):563–569. - PubMed
-
- Chen H, Chan DC. 2005. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14:R283–R289.
Publication types
MeSH terms
Substances
Grants and funding
- RC1 HL100891/HL/NHLBI NIH HHS/United States
- R01 HL105759/HL/NHLBI NIH HHS/United States
- R37 HL091102/HL/NHLBI NIH HHS/United States
- R01 HL113647/HL/NHLBI NIH HHS/United States
- R01 HL117163/HL/NHLBI NIH HHS/United States
- RC1-HL100891/HL/NHLBI NIH HHS/United States
- R21-HL102613/HL/NHLBI NIH HHS/United States
- R21-HL102714/HL/NHLBI NIH HHS/United States
- R01-HL067245/HL/NHLBI NIH HHS/United States
- R37-HL091102/HL/NHLBI NIH HHS/United States
- R21 HL102714/HL/NHLBI NIH HHS/United States
- R01 HL067245/HL/NHLBI NIH HHS/United States
- P01-HL085577/HL/NHLBI NIH HHS/United States
- P01 HL085577/HL/NHLBI NIH HHS/United States
- R21 HL104544/HL/NHLBI NIH HHS/United States
- R21 HL102613/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

