Dimers of mitochondrial ATP synthase form the permeability transition pore
- PMID: 23530243
- PMCID: PMC3625323
- DOI: 10.1073/pnas.1217823110
Dimers of mitochondrial ATP synthase form the permeability transition pore
Abstract
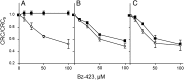
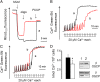
Here we define the molecular nature of the mitochondrial permeability transition pore (PTP), a key effector of cell death. The PTP is regulated by matrix cyclophilin D (CyPD), which also binds the lateral stalk of the FOF1 ATP synthase. We show that CyPD binds the oligomycin sensitivity-conferring protein subunit of the enzyme at the same site as the ATP synthase inhibitor benzodiazepine 423 (Bz-423), that Bz-423 sensitizes the PTP to Ca(2+) like CyPD itself, and that decreasing oligomycin sensitivity-conferring protein expression by RNAi increases the sensitivity of the PTP to Ca(2+). Purified dimers of the ATP synthase, which did not contain voltage-dependent anion channel or adenine nucleotide translocator, were reconstituted into lipid bilayers. In the presence of Ca(2+), addition of Bz-423 triggered opening of a channel with currents that were typical of the mitochondrial megachannel, which is the PTP electrophysiological equivalent. Channel openings were inhibited by the ATP synthase inhibitor AMP-PNP (γ-imino ATP, a nonhydrolyzable ATP analog) and Mg(2+)/ADP. These results indicate that the PTP forms from dimers of the ATP synthase.
Conflict of interest statement
Conflict of interest statement: Bz-423 is licensed to a company in which G.D.G. has ownership interest and receives compensation.
Figures



References
-
- Bernardi P, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273(10):2077–2099. - PubMed
-
- Raaflaub J. Die schwellung isolierter leberzell mitochondrien und ihre physikalisch beeinflußarkeit. Helv Physiol Pharmacol Acta. 1953;11:142–156. - PubMed
-
- Pfeiffer DR, Kuo TH, Tchen TT. Some effects of Ca2+, Mg2+, and Mn2+ on the ultrastructure, light-scattering properties, and malic enzyme activity of adrenal cortex mitochondria. Arch Biochem Biophys. 1976;176(2):556–563. - PubMed
-
- Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251(16):5069–5077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

