Synthesis of skeletally diverse and stereochemically complex library templates derived from isosteviol and steviol
- PMID: 23530630
- PMCID: PMC3638842
- DOI: 10.1021/ol400385w
Synthesis of skeletally diverse and stereochemically complex library templates derived from isosteviol and steviol
Abstract
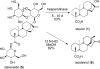
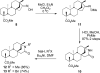
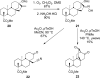
We have applied a diversity-oriented approach for the synthesis of skeletally diverse and stereochemically complex templates for small-molecule library production by performing Beckmann rearrangement and Beckmann fragmentation reactions on the bicyclo[3.2.1]octane rings of steviol and isosteviol, aglycones derived from the diterpene natural product stevioside. The optimization of these two reaction pathways is presented along with the successful application of a photo-Beckmann rearrangement. This work also led to the discovery of cyano-Prins-type and Thorpe-Ziegler-type cyclization reactions.
Figures





References
-
-
Burke MD, Berger EM, Schreiber SL. Science. 2003;302:613.Thomas GL, Wyatt EE, Spring DR. Curr. Opin. Drug. Discovery Dev. 2006;9:700.Mao S, Probst D, Werner S, Chen J, Xie X, Brummond KM. J. Comb. Chem. 2008;10:235. For a recent related approach see: Huigens RW, III, Morrison KC, Hicklin RW, Flood TA, Jr., Richter MF, Hergenrother PJ. Nat. Chem. 2013;5:195.
-
-
- Feher M, Schmidt JM. J. Chem. Inf. Comput. Sci. 2003;43:218. - PubMed
-
- Newman DJ. J. Med. Chem. 2008;51:2589. - PubMed
-
- Breinbauer R, Vetter IR, Waldmann H. Angew. Chem. Int. Ed. 2002;41:2878. - PubMed
-
- Hanson JR, De Oliveira BH. Nat. Prod. Rep. 1993;10:301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

