MEK inhibition increases lapatinib sensitivity via modulation of FOXM1
- PMID: 23531216
- PMCID: PMC3650616
- DOI: 10.2174/0929867311320190008
MEK inhibition increases lapatinib sensitivity via modulation of FOXM1
Abstract
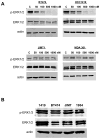
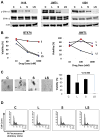
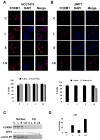
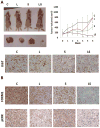
The standard targeted therapy for HER2-overexpressing breast cancer is the HER2 monoclonal antibody, trastuzumab. Although effective, many patients eventually develop trastuzumab resistance. The dual EGFR/HER2 small molecule tyrosine kinase inhibitor lapatinib is approved for use in trastuzumab-refractory metastatic HER2-positive breast cancer. However, lapatinib resistance is a problem as most patients with trastuzumab-refractory disease do not benefit from lapatinib. Understanding the mechanisms underlying lapatinib resistance may ultimately facilitate development of new therapeutic strategies for HER2-overexpressing breast cancer. Our current results indicate that MEK inhibition increases lapatinib-mediated cytotoxicity in resistant HER2-overexpressing breast cancer cells. We genetically and pharmacologically blocked MEK/ERK signaling and evaluated lapatinib response by trypan blue exclusion, anchorage-independent growth assays, flow cytometric cell cycle and apoptosis analysis, and in tumor xenografts. Combined MEK inhibition and lapatinib treatment reduced phosphorylated ERK more than single agent treatment. In addition, Western blots, immunofluorescence, and immunohistochemistry demonstrated that the combination of MEK inhibitor plus lapatinib reduced nuclear expression of the MEK/ERK downstream proto-oncogene FOXM1. Genetic knockdown of MEK was tested for the ability to increase lapatinib-mediated cell cycle arrest or apoptosis in JIMT-1 and MDA361 cells. Finally, xenograft studies demonstrated that combined pharmacological inhibition of MEK plus lapatinib suppressed tumor growth and reduced expression of FOXM1 in HER2-overexpressing breast cancers that are resistant to trastuzumab and lapatinib. Our results suggest that FoxM1 contributes to lapatinib resistance downstream of MEK signaling, and supports further study of pharmacological MEK inhibition to improve response to lapatinib in HER2-overexpressing trastuzumab-resistant breast cancer.
Conflict of interest statement
The authors declare that there are not any conflicts of interest pertaining to the work discussed in this article.
Figures




References
-
- Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Current opinion in cell biology. 2007;19:124–134. - PubMed
-
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nature reviews Molecular cell biology. 2006;7:505–516. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature reviews Molecular cell biology. 2001;2:127–137. - PubMed
-
- Eccles SA. The role of c-erbB-2/HER2/neu in breast cancer progression and metastasis. J Mammary Gland Biol Neoplasia. 2001;6:393–406. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

