Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration
- PMID: 23531334
- PMCID: PMC3616827
- DOI: 10.1186/1743-8977-10-9
Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration
Abstract
Background: The in vivo kinetics of nanoparticles is an essential to understand the hazard of nanoparticles. Here, the absorption, distribution, and excretion patterns of titanium dioxide (TiO2) and zinc oxide (ZnO) nanoparticles following oral administration were evaluated.
Methods: Nanoparticles were orally administered to rats for 13 weeks (7 days/week). Samples of blood, tissues (liver, kidneys, spleen, and brain), urine, and feces were obtained at necropsy. The level of Ti or Zn in each sample was measured using inductively coupled plasma-mass spectrometry.
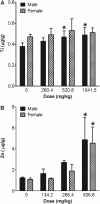
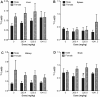
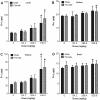
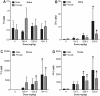
Results: TiO₂ nanoparticles had extremely low absorption, while ZnO nanoparticles had higher absorption and a clear dose-response curve. Tissue distribution data showed that TiO₂ nanoparticles were not significantly increased in sampled organs, even in the group receiving the highest dose (1041.5 mg/kg body weight). In contrast, Zn concentrations in the liver and kidney were significantly increased compared with the vehicle control. ZnO nanoparticles in the spleen and brain were minimally increased. Ti concentrations were not significantly increased in the urine, while Zn levels were significantly increased in the urine, again with a clear dose-response curve. Very high concentrations of Ti were detected in the feces, while much less Zn was detected in the feces.
Conclusions: Compared with TiO₂ nanoparticles, ZnO nanoparticles demonstrated higher absorption and more extensive organ distribution when administered orally. The higher absorption of ZnO than TiO₂ nanoparticles might be due to the higher dissolution rate in acidic gastric fluid, although more thorough studies are needed.
Figures


References
-
- Lin CC, Lin WJ. Sun protection factor analysis of sunscreens containing titanium dioxide nanoparticles. J Food Drug Anal. 2011;19(1):1–8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

