Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves
- PMID: 23531444
- PMCID: PMC4437693
- DOI: 10.1016/j.yjmcc.2013.03.010
Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves
Abstract
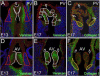
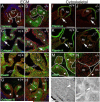
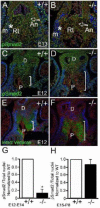
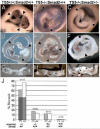
Bicuspid or bifoliate aortic valve (BAV) results in two rather than three cusps and occurs in 1-2% of the population placing them at higher risk of developing progressive aortic valve disease. Only NOTCH-1 has been linked to human BAV, and genetically modified mouse models of BAV are limited by low penetrance and additional malformations. Here we report that in the Adamts5(-/-) valves, collagen I, collagen III, and elastin were disrupted in the malformed hinge region that anchors the mature semilunar cusps and where the ADAMTS5 proteoglycan substrate versican, accumulates. ADAMTS5 deficient prevalvular mesenchyme also exhibited a reduction of α-smooth muscle actin and filamin A suggesting versican cleavage may be involved in TGFβ signaling. Subsequent evaluation showed a significant decrease of pSmad2 in regions of prevalvular mesenchyme in Adamts5(-/-) valves. To test the hypothesis that ADAMTS5 versican cleavage is required, in part, to elicit Smad2 phosphorylation we further reduced Smad2 in Adamts5(-/-) mice through intergenetic cross. The Adamts5(-/-);Smad2(+/-) mice had highly penetrant BAV and bicuspid pulmonary valve (BPV) malformations as well as increased cusp and hinge size compared to the Adamts5(-/-) and control littermates. These studies demonstrate that semilunar cusp malformations (BAV and BPV) can arise from a failure to remodel the proteoglycan-rich provisional ECM. Specifically, faulty versican clearance due to ADAMTS5 deficiency blocks the initiation of pSmad2 signaling, which is required for excavation of endocardial cushions during aortic and pulmonary valve development. Further studies using the Adamts5(-/-); Smad2(+/-) mice with highly penetrant and isolated BAV, may lead to new pharmacological treatments for valve disease.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Adamts5-/- Mice Exhibit Altered Aggrecan Proteolytic Profiles That Correlate With Ascending Aortic Anomalies.Arterioscler Thromb Vasc Biol. 2019 Oct;39(10):2067-2081. doi: 10.1161/ATVBAHA.119.313077. Epub 2019 Aug 1. Arterioscler Thromb Vasc Biol. 2019. PMID: 31366218 Free PMC article.
-
Altered versican cleavage in ADAMTS5 deficient mice; a novel etiology of myxomatous valve disease.Dev Biol. 2011 Sep 1;357(1):152-64. doi: 10.1016/j.ydbio.2011.06.041. Epub 2011 Jul 1. Dev Biol. 2011. PMID: 21749862 Free PMC article.
-
Pericellular versican regulates the fibroblast-myofibroblast transition: a role for ADAMTS5 protease-mediated proteolysis.J Biol Chem. 2011 Sep 30;286(39):34298-310. doi: 10.1074/jbc.M111.254938. Epub 2011 Aug 2. J Biol Chem. 2011. PMID: 21828051 Free PMC article.
-
Embryonic development of bicuspid aortic valves.Prog Cardiovasc Dis. 2020 Jul-Aug;63(4):407-418. doi: 10.1016/j.pcad.2020.06.008. Epub 2020 Jun 25. Prog Cardiovasc Dis. 2020. PMID: 32592706 Review.
-
Chronic inflammation: A key role in degeneration of bicuspid aortic valve.J Mol Cell Cardiol. 2019 May;130:59-64. doi: 10.1016/j.yjmcc.2019.03.013. Epub 2019 Mar 15. J Mol Cell Cardiol. 2019. PMID: 30885747 Review.
Cited by
-
Development of myotendinous-like junctions that anchor cardiac valves requires fibromodulin and lumican.Dev Dyn. 2016 Oct;245(10):1029-42. doi: 10.1002/dvdy.24435. Epub 2016 Aug 25. Dev Dyn. 2016. PMID: 27503167 Free PMC article.
-
Rhythms of growth: unveiling the mechanobiology behind heart maturation.J Physiol. 2025 Apr 11:10.1113/JP287905. doi: 10.1113/JP287905. Online ahead of print. J Physiol. 2025. PMID: 40215090 Review.
-
A unifying gene signature for adenoid cystic cancer identifies parallel MYB-dependent and MYB-independent therapeutic targets.Oncotarget. 2014 Dec 30;5(24):12528-42. doi: 10.18632/oncotarget.2985. Oncotarget. 2014. PMID: 25587024 Free PMC article.
-
Adamts5-/- Mice Exhibit Altered Aggrecan Proteolytic Profiles That Correlate With Ascending Aortic Anomalies.Arterioscler Thromb Vasc Biol. 2019 Oct;39(10):2067-2081. doi: 10.1161/ATVBAHA.119.313077. Epub 2019 Aug 1. Arterioscler Thromb Vasc Biol. 2019. PMID: 31366218 Free PMC article.
-
ADAMTS4 and ADAMTS5 knockout mice are protected from versican but not aggrecan or brevican proteolysis during spinal cord injury.Biomed Res Int. 2014;2014:693746. doi: 10.1155/2014/693746. Epub 2014 Jul 3. Biomed Res Int. 2014. PMID: 25101296 Free PMC article.
References
-
- Calloway TJ, Martin LJ, Zhang X, Tandon A, Benson DW, Hinton RB. Risk factors for aortic valve disease in bicuspid aortic valve: a family-based study. Am J Med Genet A. 2011;155A:1015–20. - PubMed
-
- Supino PG, Borer JS, Yin A, Dillingham E, McClymont W. The epidemiology of valvular heart diseases: the problem is growing. Adv Cardiol. 2004;41:9–15. - PubMed
-
- Goda M, Budts W, Troost E, Meyns B. Bicuspid Pulmonary Valve With Atrial Septal Defect Leading to Pulmonary Aneurysm. Ann Thorac Surg. 2011;93:1706–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

